
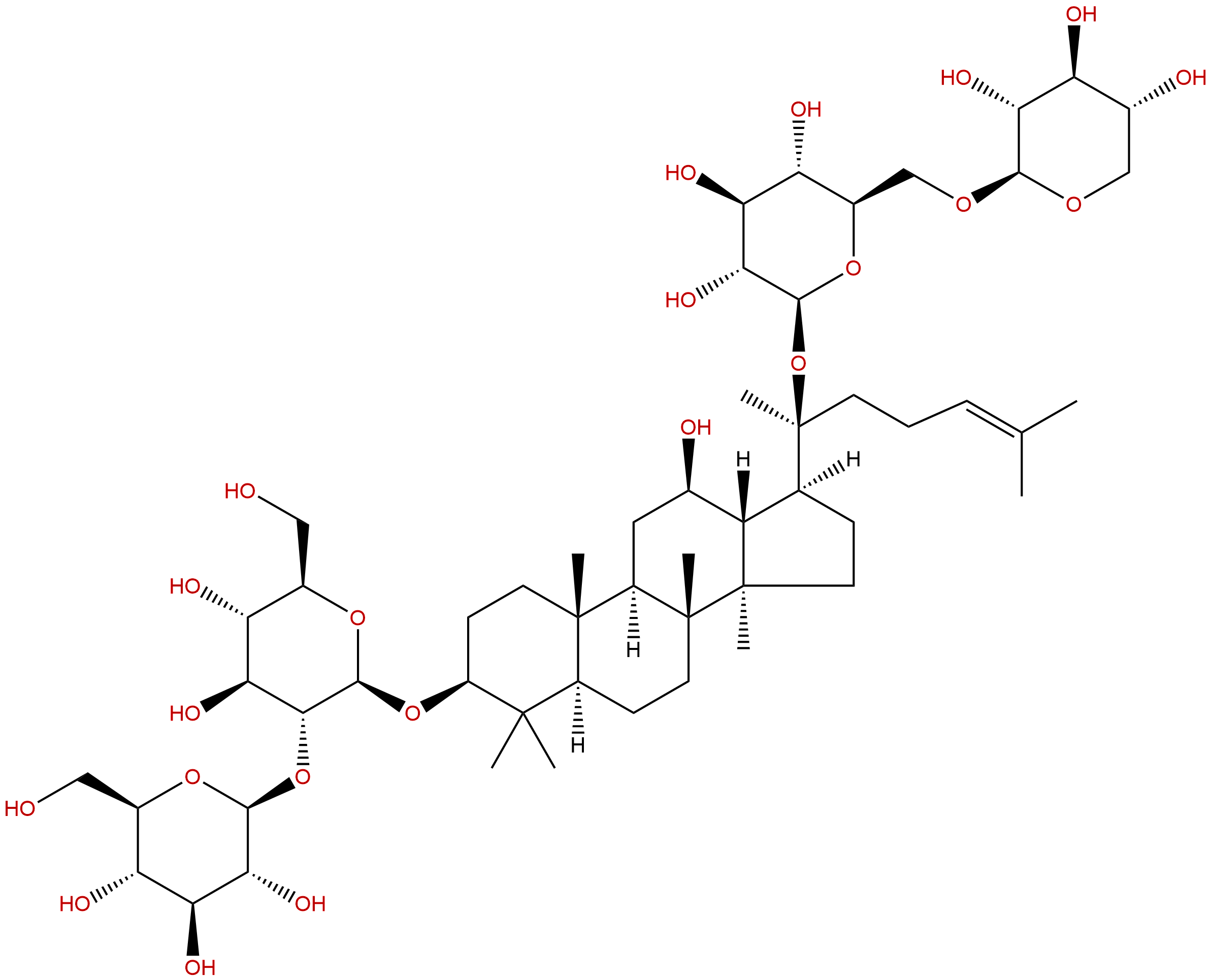
Ginsenoside Rb3CAS No.:68406-26-8
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0659 |
| Formula: | C53H90O22 |
| Mol Weight: | 1079.28 |
Product name: Ginsenoside Rb3
Synonym name: Gypenoside IV
Catalogue No.: BP0659
Cas No.: 68406-26-8
Formula: C53H90O22
Mol Weight: 1079.28
Botanical Source: Ginseng Radix Et Rhizoma
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Ginsenoside Rb3 has anti-myocardial ischemia-reperfusion injury, neuroprotective, antidepressant-like, and antioxidant effects, it also possesses the potential of the clinical use in preventing and treating diabetes. Ginsenoside Rb3 significantly attenuates the changes of creatine kinase activity and lactate dehydrogenase activity, exhibits inhibitory effect on TNFα-induced NF-κB transcriptional activity with an IC50 of 8.2 μM in 293T cell lines, it also inhibits the induction of COX-2 and iNOS mRNA.
References:
Br J Pharmacol. 2014 Jul;171(13):3171-81.
Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats.
Panax ginseng is commonly used to treat cardiovascular conditions in Oriental countries. This study investigated the mechanisms underlying the vascular benefits of Ginsenoside Rb3 (Rb3) in hypertension.
METHODS AND RESULTS:
Rings of renal arteries were prepared from spontaneously hypertensive rats (SHRs) and normotensive Wistar-Kyoto (WKY) rats and were cultured ex vivo for 8 h. Contractile responses of the rings were assessed with myograph techniques. Expression of NADPH oxidases was assessed by Western blotting and immunohistochemistry. Reactive oxygen species (ROS) were measured using dihydroethidium fluorescence imaging and production of NO was determined using the fluorescent NO indicator DAF-FM diacetate in human umbilical vein endothelial cells. Ex vivo treatment with Rb3 concentration-dependently augmented endothelium-dependent relaxations, suppressed endothelium-dependent contractions and reduced ROS production and expressions of NOX-2, NOX-4 and p67(phox) in arterial rings from SHR. Rb3 treatment also normalized angiotensin II (Ang II)-stimulated elevation in ROS and expression of NOX-2 and NOX-4 in arterial rings from WKY rats. Rb3 inhibited Ang II-induced reduction of NO production and phosphorylation of endothelial NOS in cultures of human umbilical vein endothelial cells. Rb3 also inhibited oxidative stress in renal arterial rings from hypertensive patients or in Ang II-treated arterial rings from normotensive subjects.
CONCLUSIONS:
Ex vivo Rb3 treatment restored impaired endothelial function in arterial rings from hypertensives by reversing over-expression of NADPH oxidases and over-production of ROS, and improved NO bioavailability. Our findings suggest that medicinal plants containing Rb3 could decrease oxidative stress and protect endothelial function in hypertension.
Am. Chinese Med., 2009, 37(4):759-70.
Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3.
In order to investigate the mechanisms underlying the neuroprotective effect of Ginsenoside Rb3, rat hippocampal neurons were primarily cultured, and exposed to 1 mM N-methyl-D-aspartate (NMDA), cell viability and lactate dehydrogenase leakage were measured. Ca2+ influx was determined by calcium imaging with a laser confocal microscopy.
METHODS AND RESULTS:
The influences of Ginsenoside Rb3 on these variables were examined. Patch-clamp technique was used to observe the effects of Ginsenoside Rb3 on NMDA-evoked current. The results show that treatment of Rb3 raised the neuronal viability, reduced the leakage of lactate dehydrogenase, and inhibited NMDA-elicited Ca2+ influx in a dose-dependent manner. In the presence of Rb3, NMDA-evoked peak current was inhibited, and Ca2+-induced desensitization of NMDA current was facilitated.
CONCLUSIONS:
It is suggested that Ginsenoside Rb3 could exert a neuroprotective role on hippocampal neurons, a role which was partly mediated by the facilitation of Ca2+-dependent deactivation of NMDA receptors, and the resultant reduction of intracellular free Ca2+ level.
Exp Ther Med. 2014 Dec;8(6):1751-1756.
Ginsenoside-Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis in rats.
Ginsenoside Rb3 (G-Rb3) has been previously demonstrated to attenuate myocardial ischemia-reperfusion injury (MIRI). The aim of the present study was to investigate this further and determine whether Ginsenoside Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis.
METHODS AND RESULTS:
Adult male Sprague Dawley rats were randomly divided into four groups: Sham, MIRI, Ginsenoside Rb3 treatment (orally, 20 mg/kg) and ischemic postconditioning (as the positive control). The drug or placebo treatment was administered to the rats once a day for three consecutive days, and MIRI was then induced by subjecting the rats to left anterior descending coronary artery ligation for 30 min and reperfusion for 2 h. The results showed that Ginsenoside Rb3 treatment significantly reduced the number of apoptotic cells in the myocardium and the expression of B-cell lymphoma 2-associated X protein, and increased the expression of B-cell lymphoma 2. The activities of aspartate aminotransferase, lactate dehydrogenase and creatine kinase-MB in the serum were also reduced significantly by the Ginsenoside Rb3 treatment.
CONCLUSIONS:
These findings suggest that Ginsenoside Rb3 inhibits apoptosis in the early stage of MIRI, and attenuates MIRI when the reperfusion continues. Ginsenoside Rb3 was also shown to significantly reduce the level of malondialdehyde and increase the activity of superoxide dismutase in the myocardium, which suggests that attenuating reactive oxygen species accumulation and oxidative stress may be the major mechanism underlying the anti-apoptotic effects of Ginsenoside Rb3. The release of inflammatory factors was significantly attenuated by Ginsenoside Rb3, which may also be associated with its anti-apoptotic effects.
J Psychopharmacol. 2012 May;26(5):697-713.
Ginsenoside Rb3 exerts antidepressant-like effects in several animal models.
Total ginsenosides have been shown to have therapeutic actions as antidepressants. We report a major active ingredient of total ginsenosides, the Ginsenoside Rb3 (Rb3), which may have antidepressant-like effects.
METHODS AND RESULTS:
Using the forced swim test, tail suspension test, and learned helplessness procedure, we found that Rb3 had significant anti-immobility effects in mice in the forced swim and tail suspension tests and reduced the number of escape failures in the learned helplessness procedure. In a reserpine-induced syndrome model, Rb3 attenuated hypothermia, palpebral ptosis, and akinesia. In the chronic mild stress model, chronic Rb3 administration reversed the decrease in locomotor activity, novelty-suppressed feeding, and sucrose preference. Furthermore, neurochemical tests were performed to support our hypothesis that biochemical variations (i.e. brain-derived neurotrophic factor and the monoamine neurotransmitters 5-hydroxytryptamine, dopamine, and norepinephrine) are involved in Rb3's antidepressant-like effects. Finally, we found, using whole-cell patch-clamp recordings, that the action potential transmission in neurons within the somatosensory cortex was excited by Rb3 perfusion and blocked with Panax notoginseng total saponins extracted from leaves.
CONCLUSIONS:
This study provides evidence for the mechanism of action of the antidepressant-like effects of Rb3.
HPLC of Ginsenoside Rb3

HNMR of Ginsenoside Rb3
