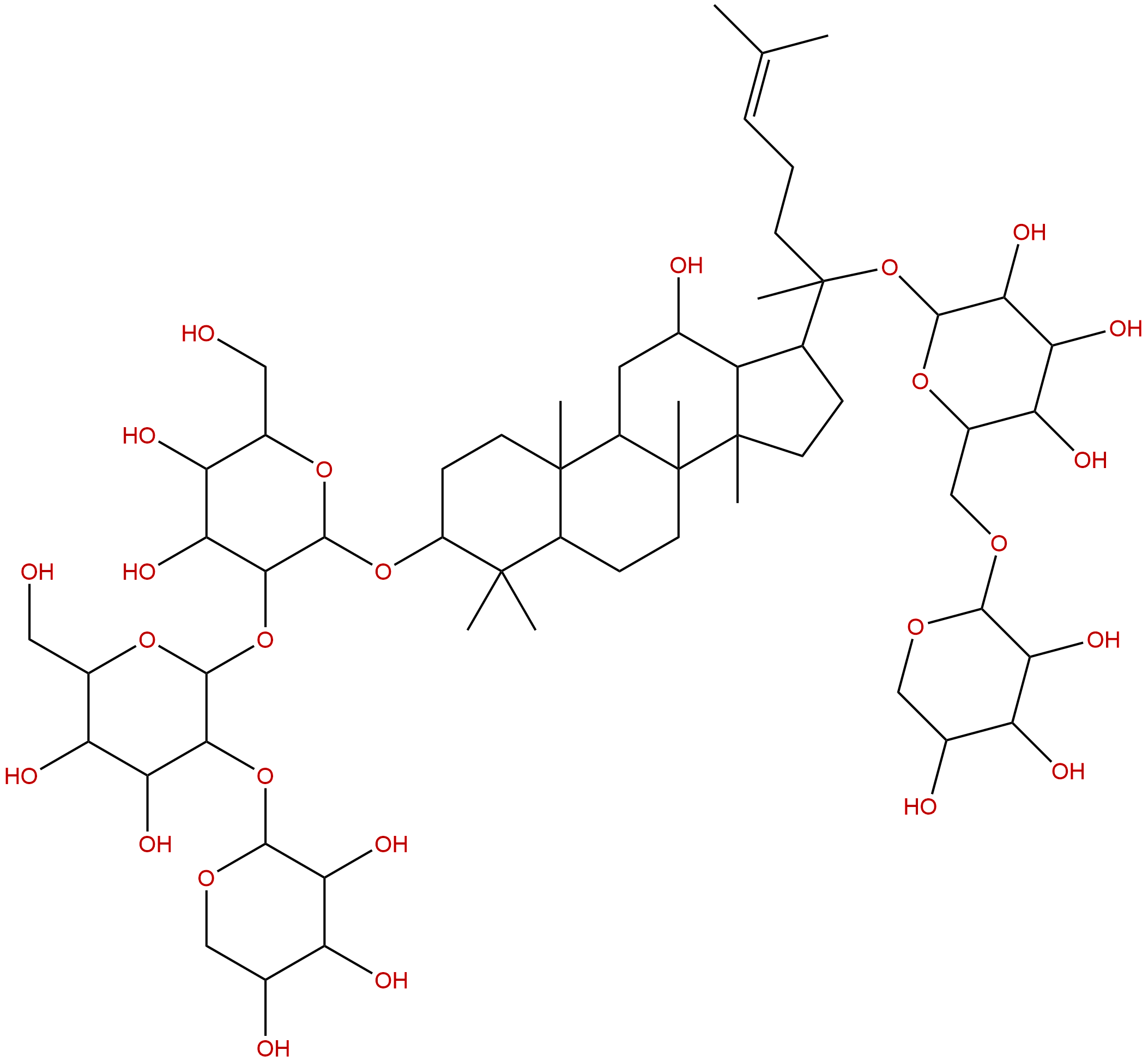
Notoginsenoside FcCAS No.:88122-52-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1008 |
| Formula: | C58H98O26 |
| Mol Weight: | 1211.4 |
Product name: Notoginsenoside Fc
Synonym name:
Catalogue No.: BP1008
Cas No.: 88122-52-5
Formula: C58H98O26
Mol Weight: 1211.4
Botanical Source: Panax notoginseng(Burk.)F.H.Chen
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Notoginsenoside Fc has perfect anti-platelet aggregatory effect.
References:
J Pharm Biomed Anal. 2015 May 10;109:150-7.
Pharmacokinetics, bioavailability, and metabolism of Notoginsenoside Fc in rats by liquid chromatography/electrospray ionization tandem mass spectrometry.
Notoginsenoside Fc (NGFc) is a protopanaxadiol-type (PPD-type) saponin from Panax notoginseng, which has perfect anti-platelet aggregatory effect. However, its pharmacokinetics and metabolism in vivo remain unknown.
METHODS AND RESULTS:
In this study, a simple and sensitive liquid chromatography/electrospray ionization tandem mass spectrometry (LC-MS/MS) method was first developed for the determination of NGFc in rat plasma. After methanol-mediated protein precipitation, separation was achieved on a C18 column with MS detection operated in negative SRM mode at m/z 604.56→m/z 783.90 and m/z 799.93→m/z 637.64 for NGFc and IS, respectively. The assay was linear over the concentration range (r>0.995) with the LLOQ of 0.002μg/ml. The intra- and inter-day precisions (R.S.D.) were 2.45-12.36% and 3.67-14.22%, respectively; whereas accuracy ranged from (R.R.) 93.90% to 99.41%. The extraction recovery, stability, and matrix effect were within the acceptable limits.
CONCLUSIONS:
The validated LC-MS/MS method was successfully applied to the pre-clinical pharmacokinetic studies of NGFc in rat. After oral and intravenous administration, NGFc showed dose-independent pharmacokinetic behaviors with a t1/2 of >22h and its oral bioavailability was 0.10-0.14%. In addition, a total of 10 metabolites were detected and structurally characterized by UPLC-Q/TOF-MS technique, which suggested that deglycosylation was the major metabolic pathway for NGFc in rats.
HPLC of Notoginsenoside Fc

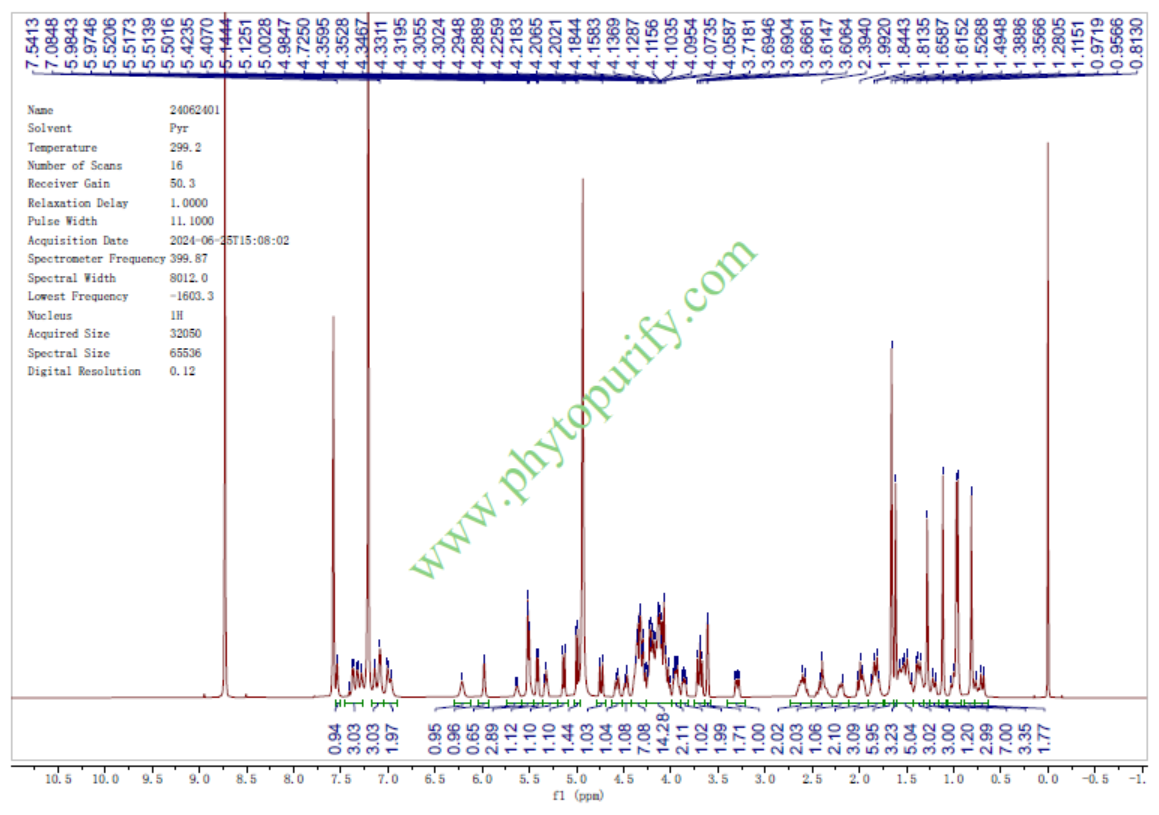
HNMR of Notoginsenoside Fc