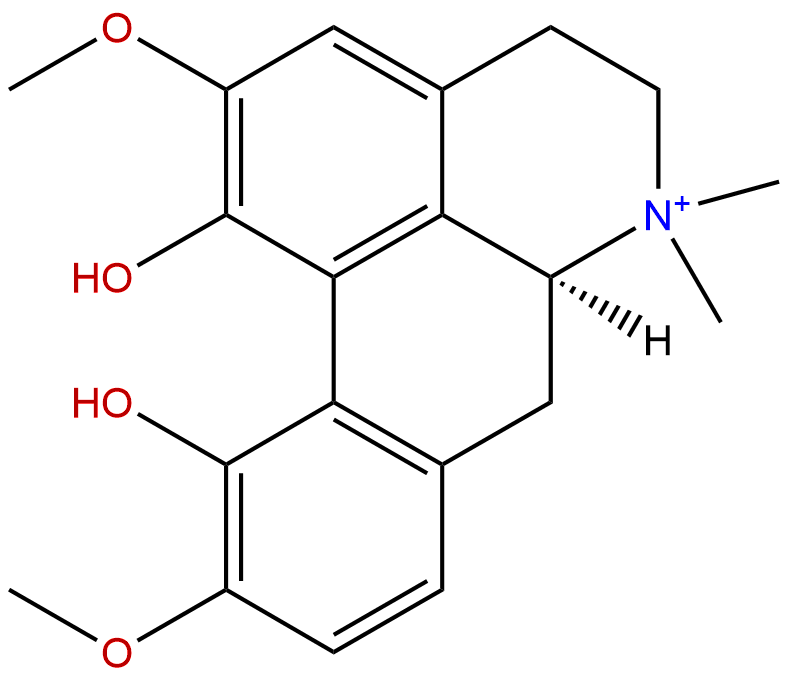
MagnoflorineCAS No.:2141-09-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0912 |
| Formula: | C20H24NO4+ |
| Mol Weight: | 342.414 |
Product name: Magnoflorine
Synonym name: Thalictrine; Esholine; Escholine; Corytuberine methosalt
Catalogue No.: BP0912
Cas No.: 2141-09-5
Formula: C20H24NO4+
Mol Weight: 342.414
Botanical Source: Magnoliae flos
Physical Description:
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Magnoflorine possesses high activity as α-glucosidase inhibitor in vitro and in vivo, has antidiabetic potential activity; it also has sedative and anxiolytic effects, probably mediated by a GABAergic mechanism of action. Magnoflorine has protective effects, mediated by some mechanism other than prevention of micelle formation or protection of the erythrocyte membrane against osmotic imbalance.
References:
J Nat Med. 2015 Apr 4.
Sinomenine and magnoflorine, major constituents of Sinomeni Caulis et Rhizoma, show potent protective effects against membrane damage induced by lysophosphatidylcholine in rat erythrocytes.
The effects of the water extract of Sinomeni Caulis et Rhizoma (SCR-WE) and its major constituents, sinomenine (SIN) and Magnoflorine (MAG), on moderate hemolysis induced by lysophosphatidylcholine (LPC) were investigated in rat erythrocytes and compared with the anti-hemolytic effects of lidocaine (LID) and propranolol (PRO) as reference drugs.
METHODS AND RESULTS:
LPC caused hemolysis at concentrations above the critical micelle concentration (CMC), and the concentration of LPC producing moderate hemolysis (60 %) was approximately 10 μM. SCR-WE at 1 ng/mL-100 μg/mL significantly inhibited the hemolysis induced by LPC. SIN and Magnoflorine attenuated LPC-induced hemolysis in a concentration-dependent manner from very low to high concentrations (1 nM-100 μM and 10 nM-100 μM, respectively). In contrast, the inhibiting effects of LID and PRO on LPC-induced hemolysis were observed at higher concentrations (1-100 μM) but not at lower concentrations (1-100 nM). Neither SIN nor Magnoflorine affected micelle formation of LPC, nor, at concentrations of 1 nM-1 μM, did they attenuate the hemolysis induced by osmotic imbalance (hypotonic hemolysis). Similarly, SCR-WE also did not modify micelle formation or hypotonic hemolysis, except at the highest concentration.
CONCLUSIONS:
These results suggest that SIN and Magnoflorine potently protect the erythrocyte membrane from LPC-induced damage and contribute to the beneficial action of SCR-WE. The protective effects of SIN and Magnoflorine are mediated by some mechanism other than prevention of micelle formation or protection of the erythrocyte membrane against osmotic imbalance.
Biol Pharm Bull. 2007 Jun;30(6):1157-60.
Magnoflorine from Coptidis Rhizoma protects high density lipoprotein during oxidant stress.
The objective of the present study was to investigate the beneficial properties of Magnoflorine, an alkaloid isolated from coptidis rhizoma, on protecting human high density lipoprotein (HDL) against lipid peroxidation.
METHODS AND RESULTS:
Magnoflorine exerts an inhibitory effect against Cu2+-induced lipid peroxidation of HDL, as showed by prolongation of lag time from 62 to 123 min at the concentration of 3.0 microM. It also inhibits the generation of thiobarbituric acid reactive substances (TBARS) in the dose-dependent manners with IC50 values of 2.3+/-0.2 microM and 6.2+/-0.5 microM since HDL oxidation mediated by either catalytic Cu2+ or thermo-labile radical initiator (AAPH), respectively. Separately, Cu2+ oxidized HDL lost the antioxidant action but the inclusion of Magnoflorine/Cu2+ oxidized HDL can protect LDL oxidation according to increasing Magnoflorine concentration.
CONCLUSIONS:
The results suggest that Magnoflorine may have a role to play in preventing the HDL oxidation.
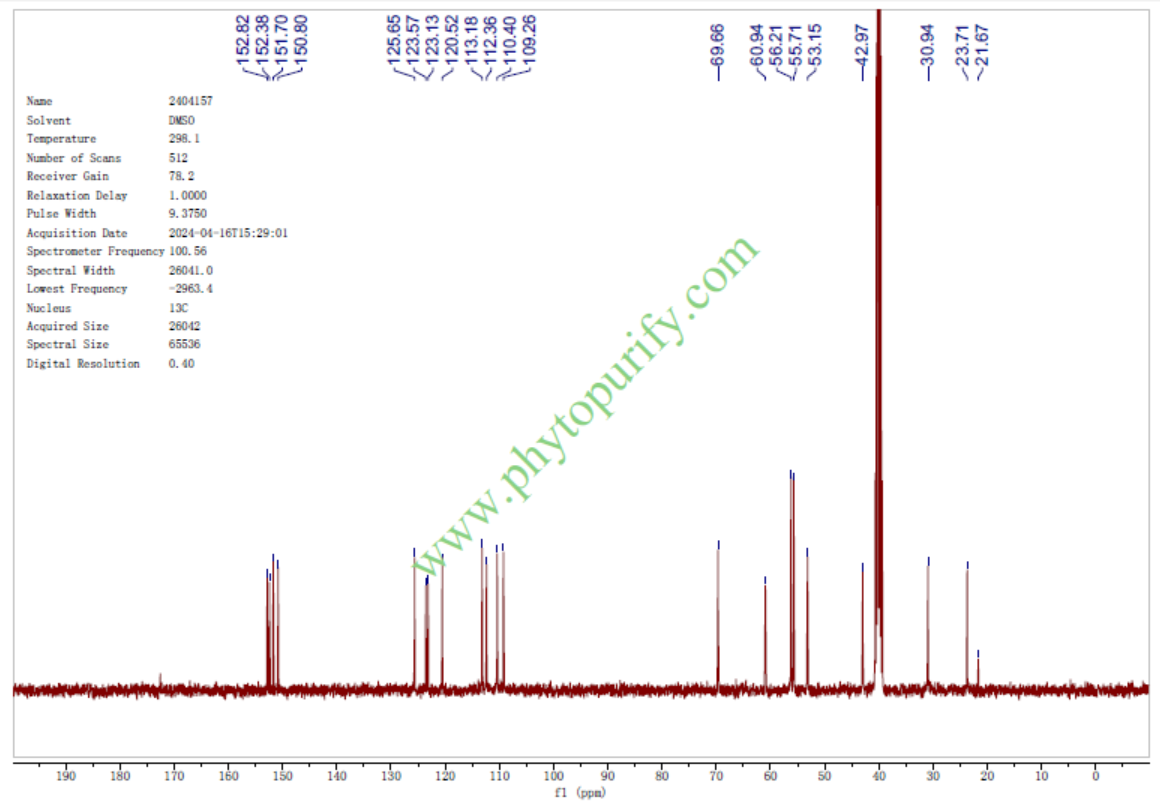
NMR of Magnoflorine
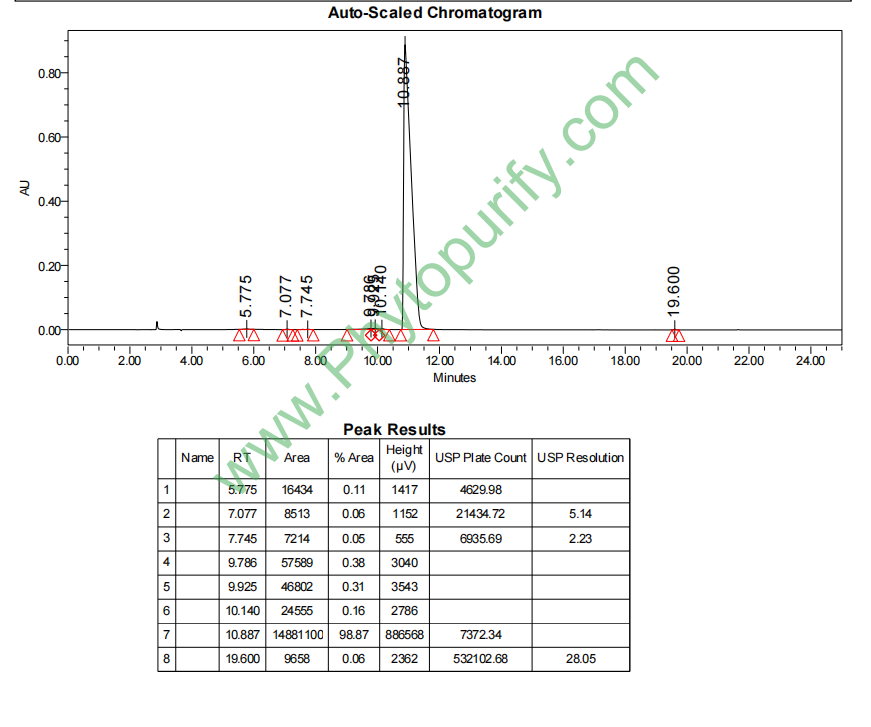
HPLC of Magnoflorine