
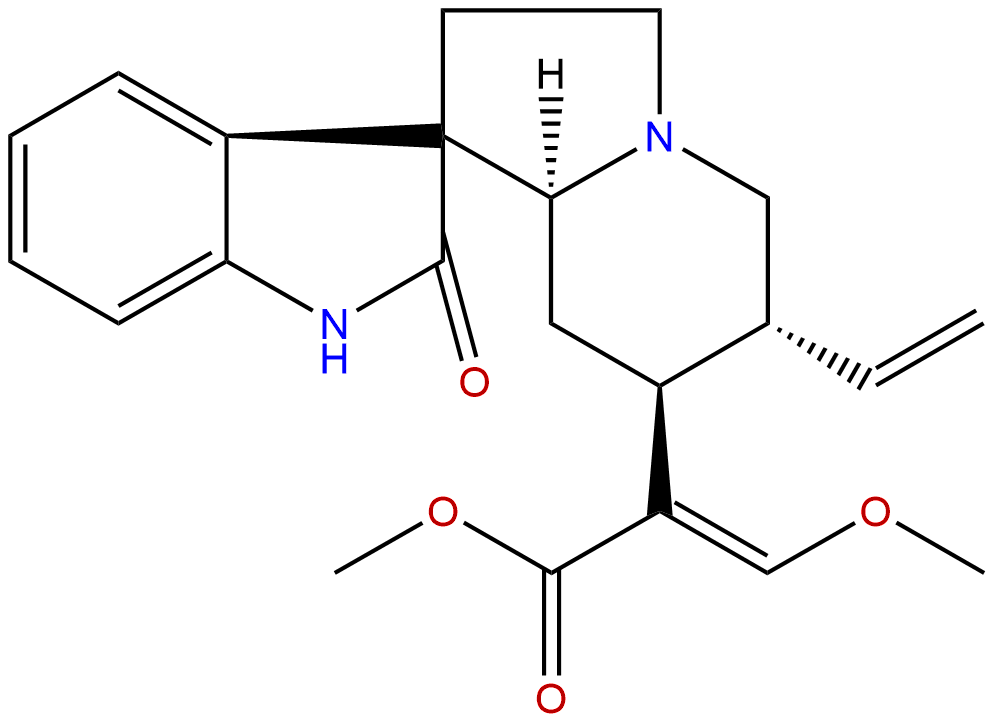
IsocorynoxeineCAS No.:51014-29-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0778 |
| Formula: | C22H26N2O4 |
| Mol Weight: | 382.46 |
Synonym name:
Catalogue No.: BP0778
Cas No.: 51014-29-0
Formula: C22H26N2O4
Mol Weight: 382.46
Botanical Source: Alkaloid from Uncaria attenuata attenuata and Mitragyna rotundifolia (Naucleaceae)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Isocorynoxeine, an isorhynchophylline-related alkaloid, exhibits a dose-dependent inhibition of 5-HT2A receptor-mediated current response with an IC50 of 72.4 μM. Isocorynoxeine shows the effects of lowering blood pressure, vasodilatation, and protection against ischemia-induced neuronal damage, it exhibits a significant neuroprotective effect against glutamate-induced HT22 cell death at the maximum concentration.
References:
Planta Med. 2015 Jan;81(1):46-55.
Isolation and identification of twelve metabolites of isocorynoxeine in rat urine and their neuroprotective activities in HT22 cell assay.
Isocorynoxeine, one of the major alkaloids from Uncaria Hook, shows the effects of lowering blood pressure, vasodilatation, and protection against ischemia-induced neuronal damage.
METHODS AND RESULTS:
In this paper, the metabolism of Isocorynoxeine was investigated in rats. Twelve metabolites and the parent drug were isolated by using solvent extraction and repeated chromatographic methods, and determined by spectroscopic methods including UV, MS, NMR, and CD experiments. Seven new compounds were identified as 11-hydroxyIsocorynoxeine, 5-oxoisocorynoxeinic acid-22-O-β-D-glucuronide, 10-hydroxyIsocorynoxeine, 17-O-demethyl-16,17-dihydro-5-oxoIsocorynoxeine, 5-oxoisocorynoxeinic acid, 21-hydroxy-5-oxoIsocorynoxeine, and oxireno[18, 19]-5-oxoIsocorynoxeine, together with six known compounds identified as Isocorynoxeine, 18,19-dehydrocorynoxinic acid, 18,19-dehydrocorynoxinic acid B, corynoxeine, Isocorynoxeine-N-oxide, and corynoxeine-N-oxide. Possible metabolic pathways of Isocorynoxeine are proposed. Furthermore, the activity assay for the parent drug and some of its metabolites showed that Isocorynoxeine exhibited a significant neuroprotective effect against glutamate-induced HT22 cell death at the maximum concentration. However, little or weak neuroprotective activities were observed for M-3, M-6, M-7, and M-10.
CONCLUSIONS:
Our present study is important to further understand their metabolic fate and disposition in humans.