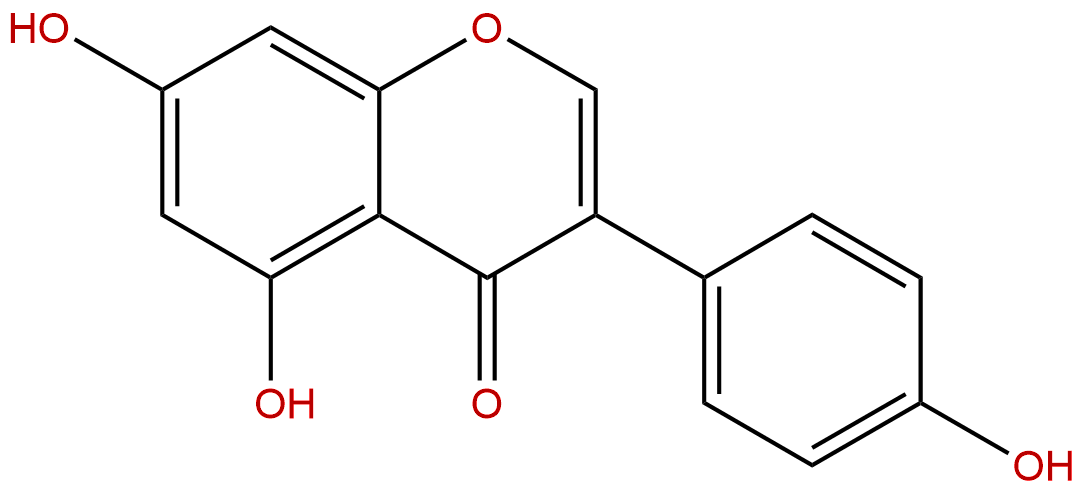
GenisteinCAS No.:446-72-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0634 |
| Formula: | C15H10O5 |
| Mol Weight: | 270.24 |
Product name: Genistein
Synonym name: 4',5,7-Trihydroxyisoflavone
Catalogue No.: BP0634
Cas No.: 446-72-0
Formula: C15H10O5
Mol Weight: 270.24
Botanical Source: V. widely distributed in the Leguminosae subf. Papilionoideae but also in Podocarpus spicatus (Podocarpaceae) and wood of Prunus spp. (Rosaceae). The major isoflavone in soy beans and isolated soy protein. Claimed to be prod. by microorganisms Streptomyc
Physical Description: Yellow powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Genistein, the major phytoestrogen in soy, is linked to diminished female reproductive performance and to cancer chemoprevention and decreased adipose deposition; genistein-induces hypermethylation persisted into adulthood, decreases ectopic Agouti expression and protecting offspring from obesity, suggests that in utero dietary genistein affects gene expression and alters susceptibility to obesity in adulthood by permanently altering the epigenome.[1]
Genistein is a specific inhibitor of tyrosine-specific protein kinases, can inhibit the EGF-stimulated increase in phosphotyrosine level in A431 cells.[2]
Genistein down-regulates NF-kappaB and phospho-in , leading to the suppression of proliferation and induction of apoptosis, thus providing the molecular basis for the treatment of patients with this pharmacologically safe agent.[3]
Genistein exerts multiple suppressive effects on human breast carcinoma cells, suggests that its mechanism of chemoprevention is pleiotropic.[4]
Genistein stimulates estrogen-responsive pS2 mRNA expression at concentrations as low as 10(-8) M and these effects can be inhibited by tamoxifen; genistein competed with [3H]estradiol binding to the estrogen receptor with 50% inhibition at 5 x 10(-7) M, thus, the estrogenic effect of genistein would appear to be a result of an interaction with the estrogen receptor.[5]
Genistein inhibits TPA-induced COX-2 expression in MCF10A cells by blocking ERK-mediated phosphorylation of p65 and its subsequent interaction with CBP and TBP.[6]
Genistein has neuroprotective effect against beta amyloid-induced neurotoxicity.[7]
References:
[1] Dolinoy D C, Weidman J R, Waterland R A, et al. Environ Heal Persp, 2006, 114(4):567-72.
[2] Akiyama T, Ishida J, Nakagawa S, et al. J Biol Chem, 1987, 262(12):5592-5.
[3] He H, Chen L, Zhai M, et al. Phytother Res, 2009, 23(6):868–73.
[4] Shao Z, Jiong W U. Int J Cancer, 1998, 22(5):362-5.
[5] Wang T T, Sathyamoorthy N, Phang J M. Carcinogenesis, 1996, 17(2):271-5.
[6] Chung M H, Kim D H, Na H K, et al. Mutat Res Fund Mol M, 2014, 768:74-83.
[7] Bang O Y, Hong H S, Dong H K, et al. Neurobiol Dis, 2004, 16(1):21-8.
[8] Zhang H, Suo Z, Zhang Z, et al. Chinese Journal of Pharmaceutical Analysis. 2003,23(1):18-9.
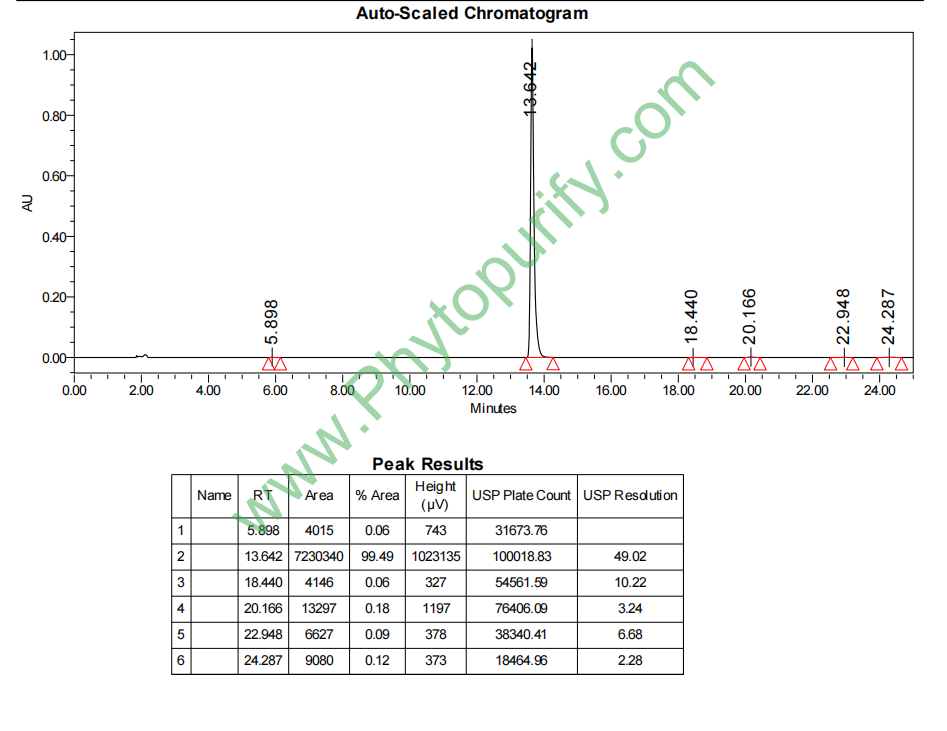
HPLC of Genistein