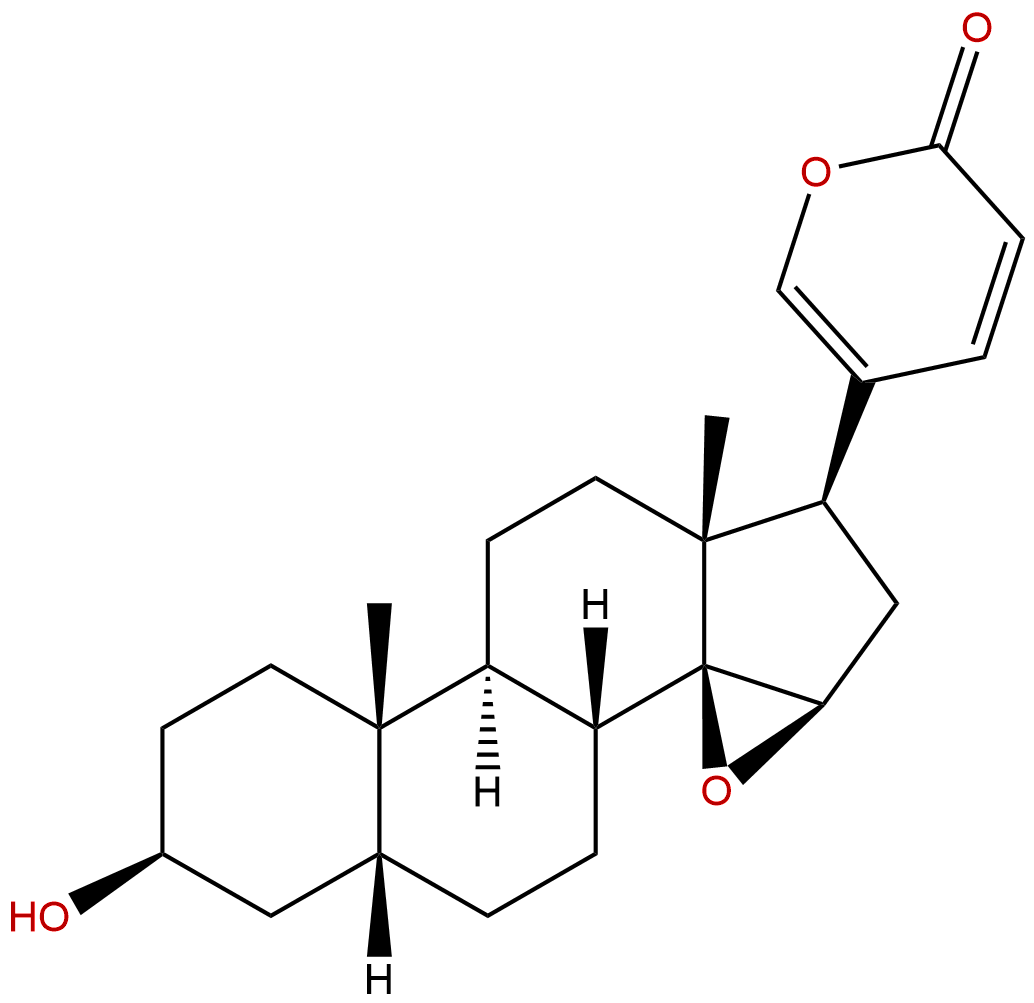
BufogeninCAS No.:465-39-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0289 |
| Formula: | C24H32O4 |
| Mol Weight: | 384.516 |
Synonym name: Resibufogenin; Respigon
Catalogue No.: BP0289
Cas No.: 465-39-4
Formula: C24H32O4
Mol Weight: 384.516
Botanical Source: toad venom extract Ch'an Su (Bufo sp.)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Resibufogenin is a cytotoxic steroid isolated from the Chinese drug ChanSu, which exhibits the anti-proliferative effect against cancer cells through the degradation of cyclin D1 caused by the activation of GSK-3β. Resibufogenin can inhibit rectifier potassium current ( I K ) and transient potassium current ( I A ), it has pathological effects on central nervous system. Resibufogenin corrects hypertension in a rat model of human preeclampsia, it not only prevents the advent of hypertension and proteinuria, but also the development of intrauterine growth restriction.
References:
Xenobiotica. 2013 May;43(5):479-85.
Isolation and identification of phase I metabolites of resibufogenin in rats.
1. Resibufogenin (1), a major bufadienolide of Chinese medicine Chan Su, had a wide range of pharmacological activities. In present work, the metabolism of 1 in male Sprague-Dawley rats was investigated by identifying the metabolites of Resibufogenin excreted in rat bile.
METHODS AND RESULTS:
2. Following an oral dose of 60 mg/kg resibufagenin, nine metabolites were isolated from bile of rats, and their structures were identified as 3-keto- Resibufogenin (2), 3-epi-Resibufogenin (3), 5β-hydroxy-3-epi-Resibufogenin (4), 1α, 5β-dihydroxy-3-epi-Resibufogenin (5), 3α, 5β, 14α, 15β-tetrahydroxyl-bufa- 20, 22-dienolide (6), 3α, 14α, 15β-trihydroxy-bufa-20, 22-dienolide (7), 3-epi- 5β-hydroxy-bufalin (8), 12α, 16β-dihydroxy-3-epi-Resibufogenin (9), and 5β, 16β-dihydroxy-3-epi-Resibufogenin (10), respectively, on the basis of widely spectroscopic methods including 2D-NMR technology. It is first time to describe the metabolites of 1 in vivo, and metabolites 5-7 and 9-10 are novel.
CONCLUSIONS:
3. On the basis of these identified metabolites, a possible metabolism pathway for 1 in rats has been proposed. This is the first systematic study on the phase I metabolites of Resibufogenin.
Hypertens Pregnancy. 2010 Jan;29(1):1-9.
Resibufogenin prevents the manifestations of preeclampsia in an animal model of the syndrome.
We have developed a rat model of preeclampsia which is based upon excessive volume expansion and includes hypertension, proteinuria and intrauterine growth restriction. In this model, the urinary excretion of the circulating steroid inhibitor of Na +/ K+ ATPase, marinobufagenin, is increased prior to the development of hypertension and proteinuria. An analogue of marinobufagenin, Resibufogenin, successfully treats the hypertension and proteinuria.
METHODS AND RESULTS:
We administered Resibufogenin early in pregnancy in this model, prior to the development of the syndrome. We found that Resibufogenin not only prevented the advent of hypertension and proteinuria, but also the development of intrauterine growth restriction.
CONCLUSIONS:
These results may have relevance to the human condition.
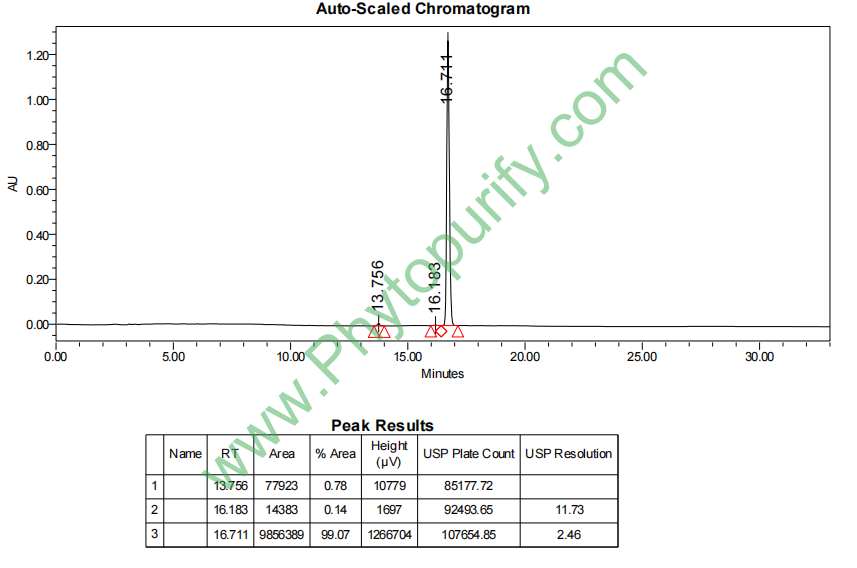
HPLC of Bufogenin