
BavachininCAS No.:19879-30-2
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0240 |
| Formula: | C21H22O4 |
| Mol Weight: | 338.403 |
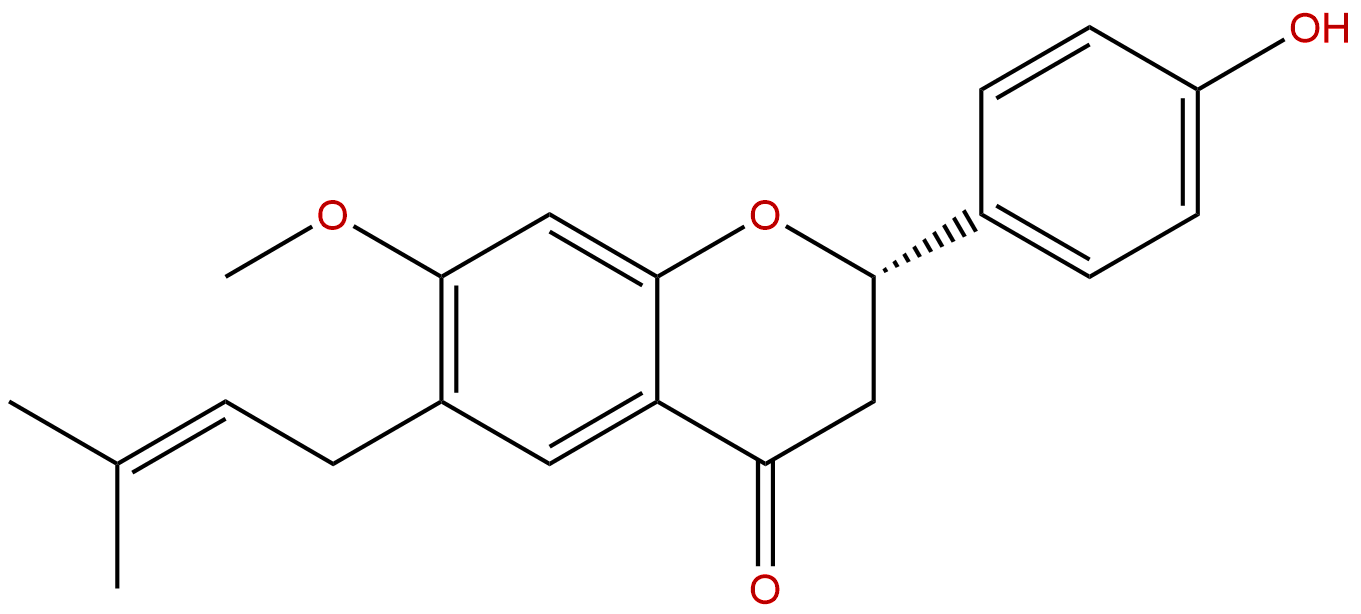
Product Name: Bavachinin
Synonym name: Bavachinin A; 7-O-Methylbavachin; 4′-Hydroxy-7-methoxy-6-prenylflavanone
Catalogue No.: BP0240
Cas No.: 19879-30-2
Formula: C21H22O4
Mol Weight: 338.403
Botanical Source: Psoralea corylifolia
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Bavachinin is a novel natural pan-PPAR agonist , it shows stronger activities with PPAR-γ than with PPAR-α and PPAR-β/δ (EC50 = 0.74 μmol/l, 4.00 μmol/l and 8.07 μmol/l in 293T cells, respectively). Bavachinin possesses anti-asthma, anti-angiogenic , anti-inflammatory, antipyretic and analgesic properties, it also exhibits glucose-lowering properties without inducing weight gain and hepatotoxicity.
References:
Indian J. Exp. Biol., 1978, 16(11):1216-7.
Antiinflamatory, antipyretic & analgesic properties of bavachinin-a flavanone isolated from seeds of Psoralea corylifolia Linn. (Babchi).
Antiinflamatory, antipyretic & analgesic properties of Bavachinin-a flavanone isolated from seeds of Psoralea corylifolia Linn. (Babchi).
Eur J Pharmacol. 2012 Sep 15;691(1-3):28-37.
Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1α.
Hypoxia-inducible factor-1 (HIF-1) consists of two subunits, the HIF-1β, which is constitutively expressed, and HIF-1α, which is oxygen-responsive. HIF-1α is over-expressed in response to hypoxia, increasing transcriptional activity linked to tumor progression, angiogenesis, metastasis, and invasion. This study aimed to demonstrate that the natural compound, Bavachinin, has potent anti-angiogenic activity in vitro and in vivo.
METHODS AND RESULTS:
Bavachinin inhibited increases in HIF-1α activity in human KB carcinoma (HeLa cell derivative) and human HOS osteosarcoma cells under hypoxia in a concentration-dependent manner, probably by enhancing the interaction between von Hippel-Lindau (VHL) and HIF-1α. Furthermore, Bavachinin decreased transcription of genes associated with angiogenesis and energy metabolism that are regulated by HIF-1, such as vascular endothelial growth factors (VEGF), Glut 1 and Hexokinase 2. Bavachinin also inhibited tube formation in human umbilical vein endothelial cells (HUVECs) as well as in vitro migration of KB cells. In vivo studies showed that injecting Bavachinin thrice weekly for four weeks significantly reduced tumor volume and CD31 expression in nude mice with KB xenografts.
CONCLUSIONS:
These data indicate that Bavachinin could be used as a therapeutic agent for inhibiting tumor angiogenesis.
HPLC of Bavachinin
