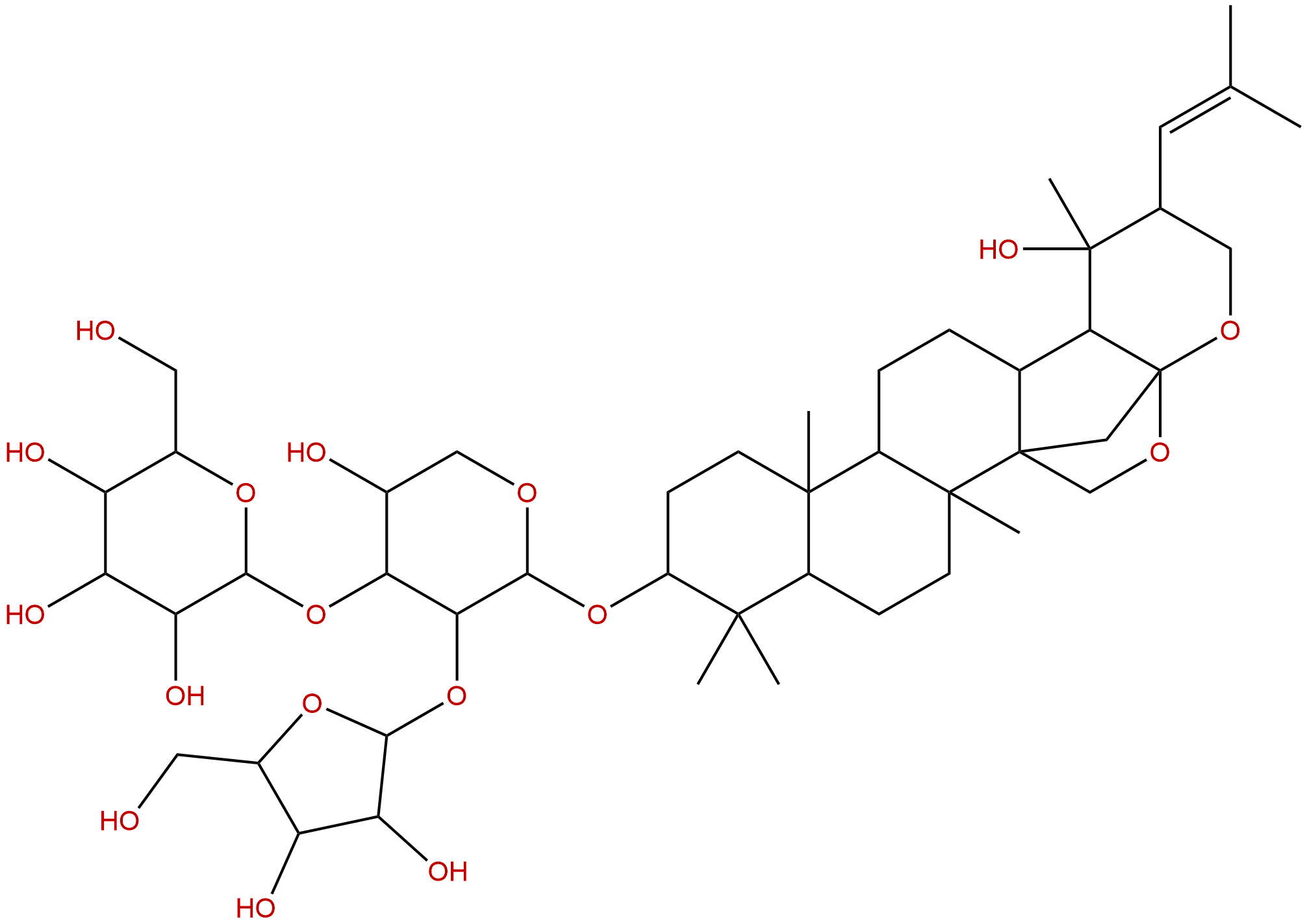
Bacopasaponin CCAS No.:178064-13-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0226 |
| Formula: | C46H74O17 |
| Mol Weight: | 899.081 |
Synonym name:
Catalogue No.: BP0226
Cas No.: 178064-13-6
Formula: C46H74O17
Mol Weight: 899.081
Botanical Source: Bacopa monnieri
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Bacopasaponin C may have antioxidant activity, it also shows anti-leishmanial property.
References:
Curr Neuropharmacol. 2018 Apr 19.
Insights into the molecular aspects of neuroprotective Bacoside A and Bacopaside I.
Bacopa monnieri, commonly known as Brahmi, has been extensively used as a neuromedicine for various disorders such as anxiety, depression and memory loss.
METHODS AND RESULTS:
Chemical characterization studies revealed the major active constituents of the herb as the triterpenoid saponins, bacosides. Bacoside A, the vital neuroprotective constituent, is composed of four constituents viz., bacoside A3, bacopaside II, jujubogenin isomer of Bacopasaponin C (bacopaside X) and Bacopasaponin C. B. monnieri extracts as well as bacosides successfully establish a healthy antioxidant environment in various tissues especially in liver and brain. Free radical scavenging, suppression of lipid peroxidation and activation of antioxidant enzymes by bacosides help to attain a physiological state of minimized oxidative stress. The molecular basis of neuroprotective activity of bacosides is attributed to the regulation of mRNA translation and surface expression of neuroreceptors such as AMPAR, NMDAR and GABAR in the various parts of the brain. Bioavailability as well as binding of neuroprotective agents (such as bacosides) to these receptors is controlled by the Blood Brain Barrier (BBB). However, nano conversion of these drug candidates easily resolves the BBB restriction and carries a promising role in future therapies.
CONCLUSIONS:
This review summarizes the neuroprotective functions of the B. monnieri extracts as well as its active compounds (bacoside A, bacopaside I) and the molecular mechanisms responsible for these pharmacological activities.
Phytother Res. 2010 Jun;24(6):864-8.
Antitumor activities of dammarane triterpene saponins from Bacopa monniera.
Bioassay-guided methods were used to test the antitumor activity of methanol extract of the whole plant of Bacopa monniera (L.) Wettst. and four different fractions (petroleum ether, CHCl(3), EtOAc, and n-BuOH fractions) of the methanol extract.
METHODS AND RESULTS:
Among the five crude samples, n-BuOH fraction was noted to have the highest antitumor activity. The dammarane triterpene saponins isolated from n-BuOH fraction, bacopaside E (1) and bacopaside VII (3), had potential antitumor effect. 1 and 3 showed cytotoxicity of all the tested human tumor cell lines MDA-MB-231, SHG-44, HCT-8, A-549 and PC-3M in MTT assay in vitro, and showed 90.52 % and 84.13 % inhibition in mouse implanted with sarcoma S180 in vivo at the concentration of 50 micromol/kg, respectively. The remaining two compounds, bacopaside II (2) and Bacopasaponin C (4) were found to be much less potent compared with 1 and 3. 1 and 3 significantly inhibited human breast cancer cell line MDA-MB-231 adhesion, migration and Matrigel invasion in vitro at the concentration of 50 micromol/L.
CONCLUSIONS:
Since no antitumor activities about the monomers from Bacopa monniera (L.) Wettst. have been reported, these results indicate that the mechanism of action of 1 and 3 needs further study.
Heliyon. 2016 Feb 15;2(2):e00068.
Beneficial effects of Bacopa monnieri extract on opioid induced toxicity.
The present study examined the hepatotoxicity and nephrotoxicity of morphine and illicit street heroin and their amelioration by a standardized methanolic extract of Bacopa monnieri (L.) (mBME) in rats.
METHODS AND RESULTS:
Morphine or street heroin was administered at a dose of 20 mg/kg for 14 and 21 days. mBME (40 mg/kg) or ascorbic acid (50 mg/kg) was administered two hours before morphine or street heroin. High performance liquid chromatography (HPLC) was used for the standardization of bacoside-A major components in mBME. The antioxidant potential of mBME was evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Administration of morphine and street heroin resulted in marked elevation of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatinine. Histopathological changes induced by morphine and street heroin after 14 days were of reversible nature while treatment for 21 days was associated with irreversible changes. Pretreatment with mBME or ascorbic acid restored the elevation of serum ALT, AST and creatinine and protected liver and kidneys from the toxicological influence of morphine and street heroin. HPLC analysis showed that mBME contained bacoside-A major components i.e. bacoside-A3 (37.5 μg/mg), bacopaside-II (4.62 μg/mg) and Bacopasaponin C (1.91 μg/mg). The EC50 for the DPPH free radical scavenging assay revealed that mBME possessed strong antioxidant potential.
CONCLUSIONS:
These results concluded that as compared to morphine, street heroin was associated with severe biochemical and histopathological changes in the liver and kidneys. Bacopa monnieri having strong antioxidant potential may provide a beneficial herbal remedy for the efficient management of opioid related hepatotoxicity and nephrotoxicity.
HPLC of Bacopasaponin C

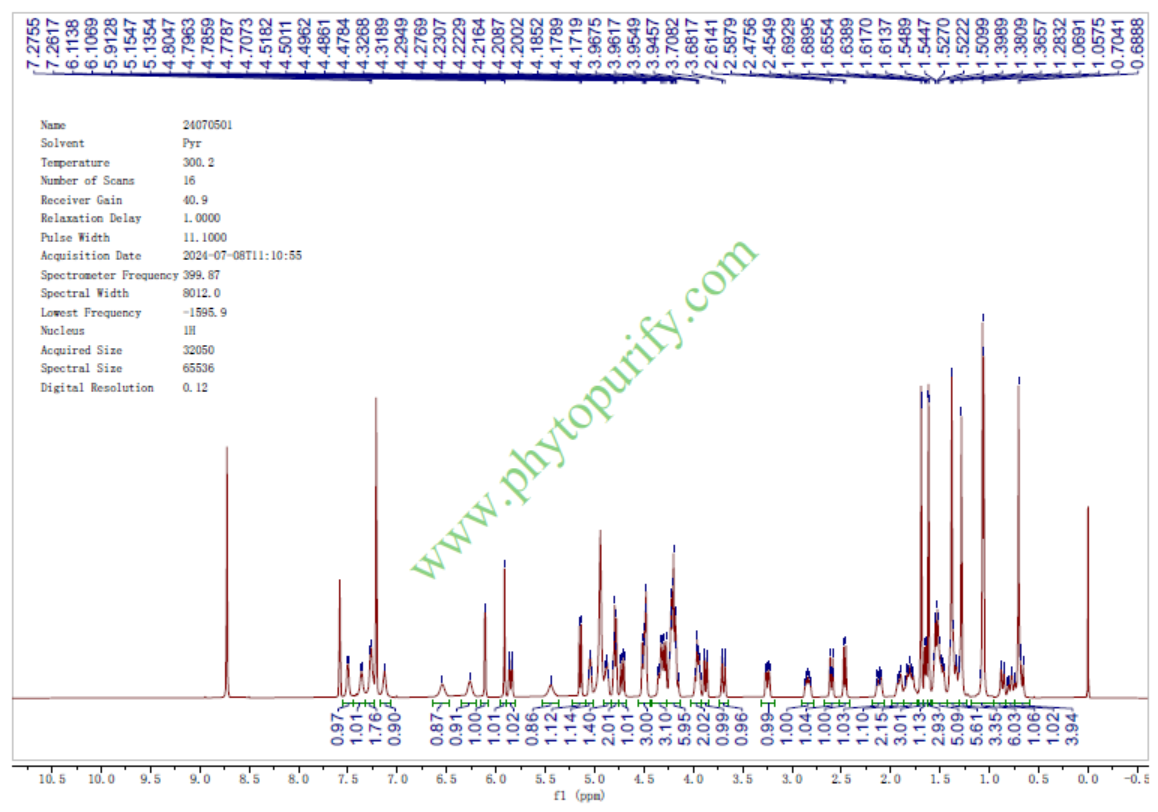
HNMR of Bacopasaponin C