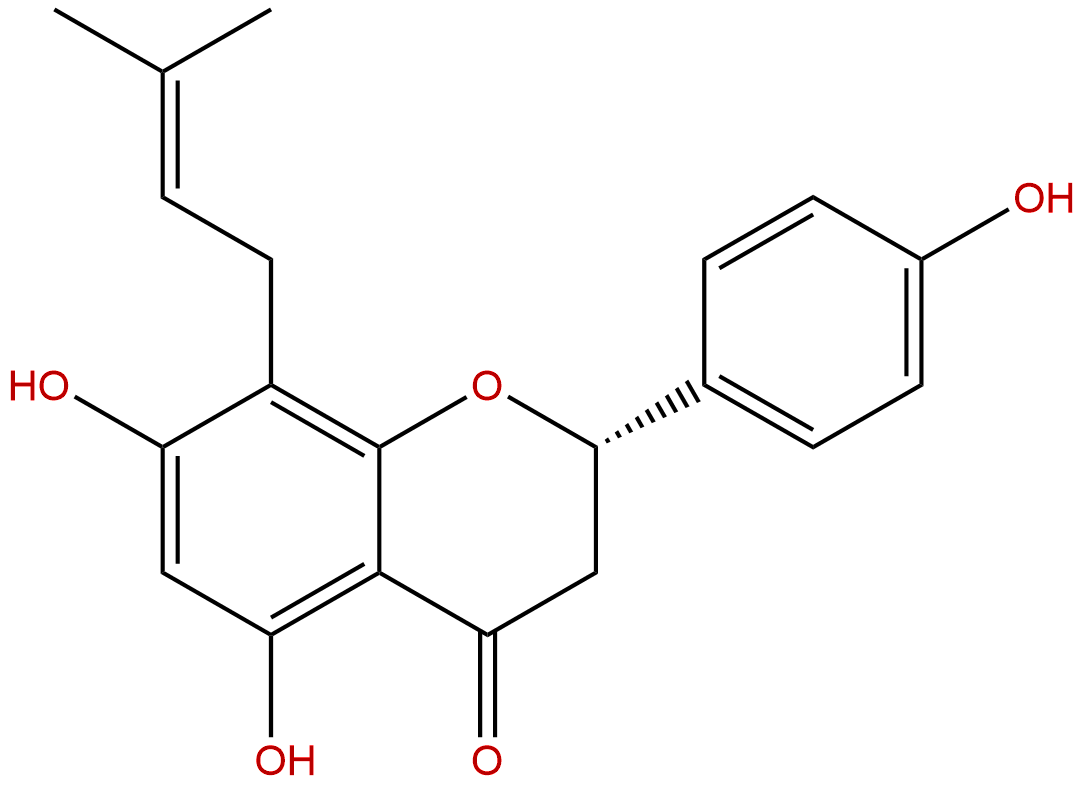
8-PrenylnaringeninCAS No.:53846-50-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | SBP02804 |
| Formula: | C20H20O5 |
| Mol Weight: | 340.375 |
Product name: 8-Prenylnaringenin
Synonym name: (+/-)-8-Prenylnaringenin,68682-02-0
Catalogue No.: SBP02804
Cas No.: 53846-50-7
Formula: C20H20O5
Mol Weight: 340.375
Botanical Source:
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
8-Prenylnaringenin is a phytoestrogen with high estrogenic activity, it shows more potent effects on promoting osteoblastic bone formation and inhibiting osteoclastic bone resorption by ERα instead of ERβ than the two classic phytoestrogens: genistein and daidzein. 8-Prenylnaringenin at all assayed doses (0.001-20 µM) presumably improves mitochondrial function, whereas a high dose of XN (5 µM) worsens the functionality of this organelle.
References:
Strahlenther Onkol. 2015 May;191(5):429-36.
8-prenylnaringenin and tamoxifen inhibit the shedding of irradiated epithelial cells and increase the latency period of radiation-induced oral mucositis : cell culture and murine model.
The major component in the pathogenesis of oral radiation-induced mucositis is progressive epithelial hypoplasia and eventual ulceration. Irradiation inhibits cell proliferation, while cell loss at the surface continues. We conceived to slow down this desquamation by increasing intercellular adhesion, regulated by the E-cadherin/catenin complex. We investigated if 8-Prenylnaringenin (8-PN) or tamoxifen (TAM) decrease the shedding of irradiated human buccal epithelial cells in vitro and thus delay the ulcerative phase of radiation-induced mucositis in vivo.
METHODS AND RESULTS:
In vitro, aggregates of buccal epithelial cells were irradiated and cultured in suspension for 11 days. 8-PN or TAM were investigated regarding their effect on cell shedding. In vivo, the lower tongue surface of mice was irradiated with graded single doses of 25 kV X-rays. The incidence, latency, and duration of the resulting mucosal ulcerations were analyzed after topical treatment with 8-PN, TAM or solvent. 8-PN or TAM prevented the volume reduction of the irradiated cell aggregates during the incubation period. This was the result of a higher residual cell number in the treated versus the untreated irradiated aggregates. In vivo, topical treatment with 8-PN or TAM significantly increased the latency of mucositis from 10.9 to 12.1 and 12.4 days respectively, while the ulcer incidence was unchanged.
CONCLUSIONS:
8-PN and TAM prevent volume reduction of irradiated cell aggregates in suspension culture. In the tongues of mice, these compounds increase the latency period. This suggests a role for these compounds for the amelioration of radiation-induced mucositis in the treatment of head and neck tumors.
Planta Med. 2015 Mar;81(4):305-11.
Neurodifferentiating potential of 8-prenylnaringenin and related compounds in neural precursor cells and correlation with estrogen-like activity.
Neurodegenerative diseases are an increasing burden for our ageing societies; there is an as yet unmet need for the development of effective therapies. Neurogenesis, i.e., the generation of new neurons in the adult brain from neural stem cells, has received increasing attention since it offers the potential for endogenous brain repair and functional regeneration. Adult neurogenesis is partially under the control of sex hormones such as estradiol, and boosting neurogenesis with estradiol in animals correlates with cognitive improvement. 8-Prenylnaringenin imitates as highly potent phytoestrogen the effects of estradiol.
METHODS AND RESULTS:
Here, we studied the potential of 8-Prenylnaringenin, 6-prenylnaringenin, and related compounds on differentiation induction in vitro using neural precursor cells transiently transfected with a doublecortin promoter luciferase construct, which was recently shown to indicate neuronal fate and differentiation. The flavanones 8-Prenylnaringenin and 6-prenylnaringenin showed slight activity in this assay but significant activity by immunostaining. Although the estrogen-like activities of 8-Prenylnaringenin and 6-prenylnaringenin are very different, the activity in differentiation induction is similar. Interestingly, also some prenylflavonoids with extended prenyl groups, e.g., a geranyl group, showed increased differentiation activity, while estrogen-like activity is decreased.
CONCLUSIONS:
This allows the conclusion that estrogen-like activity of prenylflavanones does not correlate directly with the activity of differentiation induction in neural precursor cells.
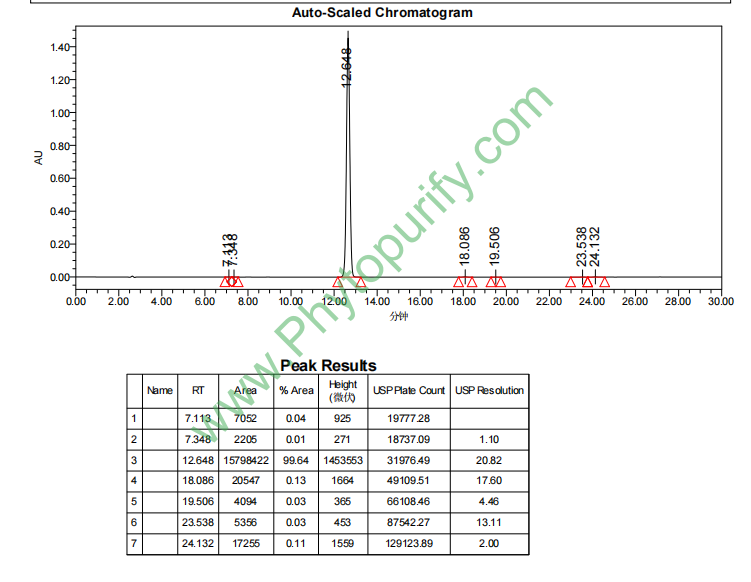
HPLC of 8-Prenylnaringenin
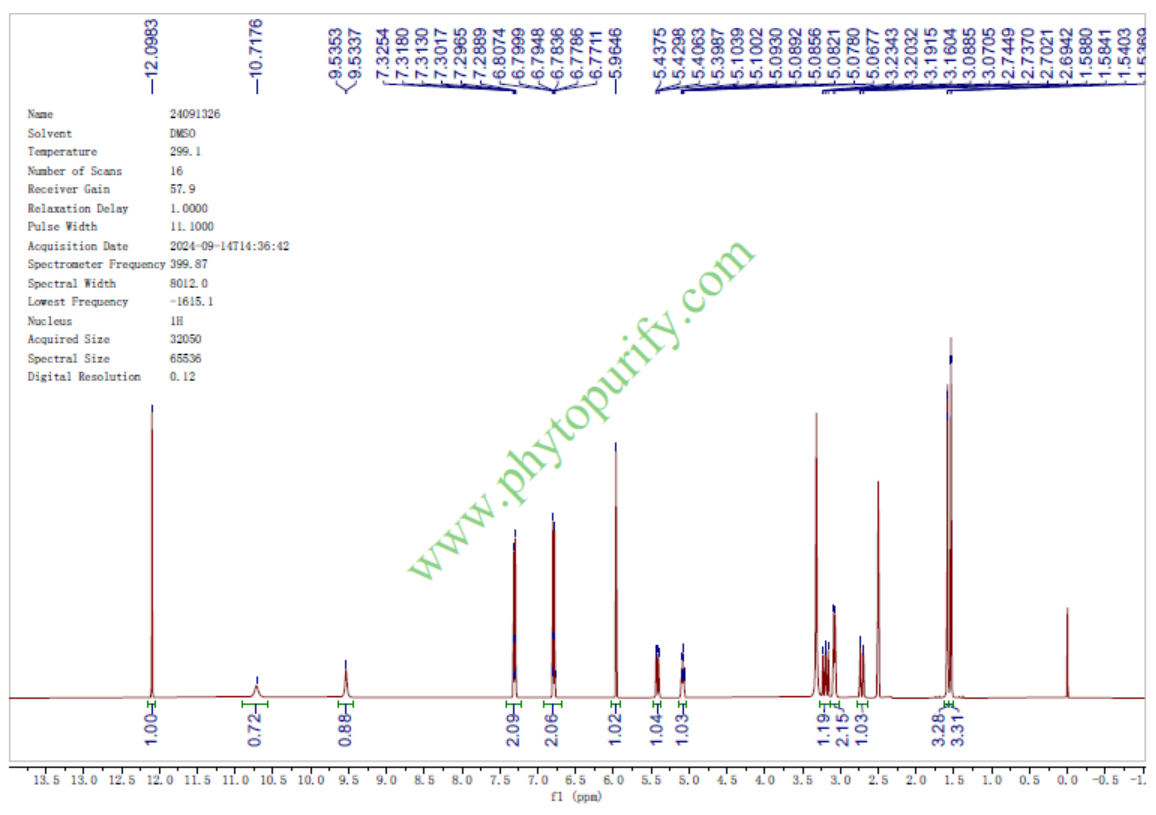
HNMR of 8-Prenylnaringenin