
MareinCAS No.:535-96-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP2136 |
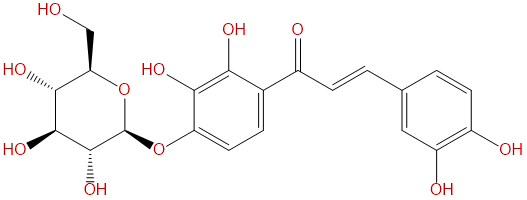
| Formula: | C21H22O11 |
| Mol Weight: | 450.396 |
Product name: Marein
Synonym name: Okanin 4'-O-β-D-Glucopyranoside
Catalogue No.: BP2136
Cas No.: 535-96-6
Formula: C21H22O11
Mol Weight: 450.396
Botanical Source: Coreopsis tinctoria Nutt.
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Marein shows neuroprotective effect on PC12 cell damage induced by methylglyoxal, which is due to a reduction of damage to mitochondria function and activation of the AMPK signal pathway, it may be a potent compound for preventing/counteracting diabetic encephalopathy. Marein can improve insulin resistance induced by high glucose in HepG2 cells through CaMKK/AMPK/GLUT1 to promote glucose uptake, through IRS/Akt/GSK-3β to increase glycogen synthesis, and through Akt/FoxO1 to decrease gluconeogenesis. Marein shows antioxidant activity, it shows Histone deacetylase enzymes (HDACs) inhibitory activity and it also can inhibit TNFα-induced NF-κB activation.
References:
Free Radic Res. 2016;50(11):1173-1187.
Marein protects against methylglyoxal-induced apoptosis by activating the AMPK pathway in PC12 cells.
Diabetic encephalopathy, which is characterized by cognitive decline and dementia, commonly occurs in patients with long-standing diabetes. Previous studies have suggested that methylglyoxal (MG), an endogenous toxic compound, plays an important role in diabetic complications such as cognitive impairment. MG induces neuronal apoptosis.
METHODS AND RESULTS:
To clarify whether Marein, a major compound from the hypoglycemic plant Coreopsis tinctoria, prevents PC12 cell damage induced by MG, we cultured PC12 cells in the presence of MG and Marein. Marein attenuated MG-induced changes in the mitochondrial membrane potential (ΔΨm), mitochondrial permeability transition pores (mPTPs), intracellular Ca2+ levels, the production of reactive oxygen species (ROS), glutathione (GSH)/glutathione disulfide (GSSG) and adenosine triphosphate (ATP), and the increase in the percentage of apoptotic cells. Marein also increased glyoxalase I (Glo1) activity, phospho-AMPKα (Thr172) and Bcl-2 expression and diminished the activation of Bax, caspase-3 and inhibitor of caspase-activated deoxyribonuclease (ICAD). Importantly, pretreatment of cells with Marein diminished the compound C-induced inactivation of p-AMPK. Molecular docking simulation showed that Marein interacted with the γ subunit of AMPK.
CONCLUSIONS:
In conclusion, we found for the first time that the neuroprotective effect of Marein is due to a reduction of damage to mitochondria function and activation of the AMPK signal pathway. These results indicate that Marein may be a potent compound for preventing/counteracting diabetic encephalopathy.
J Food Sci. 2016 Sep;81(9):C2218-23.
Rapid Identification and Comparison of Compounds with Antioxidant Activity in Coreopsis tinctoria Herbal Tea by High-Performance Thin-Layer Chromatography Coupled with DPPH Bioautography and Densitometry.
A simple and efficient method based on high-performance thin-layer chromatography coupled with 2,2-diphenyl-1-picrylhydrazyl (DPPH) bioautography (HPTLC-DPPH) was established for the screening and comparison of antioxidants in different parts of Coreopsis tinctoria herbal tea from different origins and other related herbal tea materials, which used Chrysanthemum morifolium cv. "Gongju" and "Hangju" in this study.
METHODS AND RESULTS:
Scanning densitometry after DPPH derivatization was applied for the determination of antioxidant capacities of isolated compounds in each sample. It is considered that ethanol extracts of C. tinctoria had stronger antioxidant activity and more characteristic bands than those of 2 compared samples, C. morifolium cv. "Gongju" and "Hangju." Chemometric analysis results showed that the combination of hierarchical clustering analysis and principal component analysis based on determined antioxidant capacities could be used for the discrimination of different parts of C. tinctoria and C. morifolium. Results showed that 7 compounds made up the major contributions of antioxidant activity in C. tinctoria, including okanin, isookanin, Marein, flavanoMarein, 5,7,3',5'-tetrahydroxyflavanone-7-O-glucoside, 3,5-dicaffeoylquinic acid, and chlorogenic acid. Therefore, 7 compounds were identified as major antioxidant biomarkers for quality control of C. tinctoria.
CONCLUSIONS:
Results demonstrated that the established method could be applied for the identification of C. tinctoria, and were beneficial for the bioactivity-based quality control of C. tinctoria.