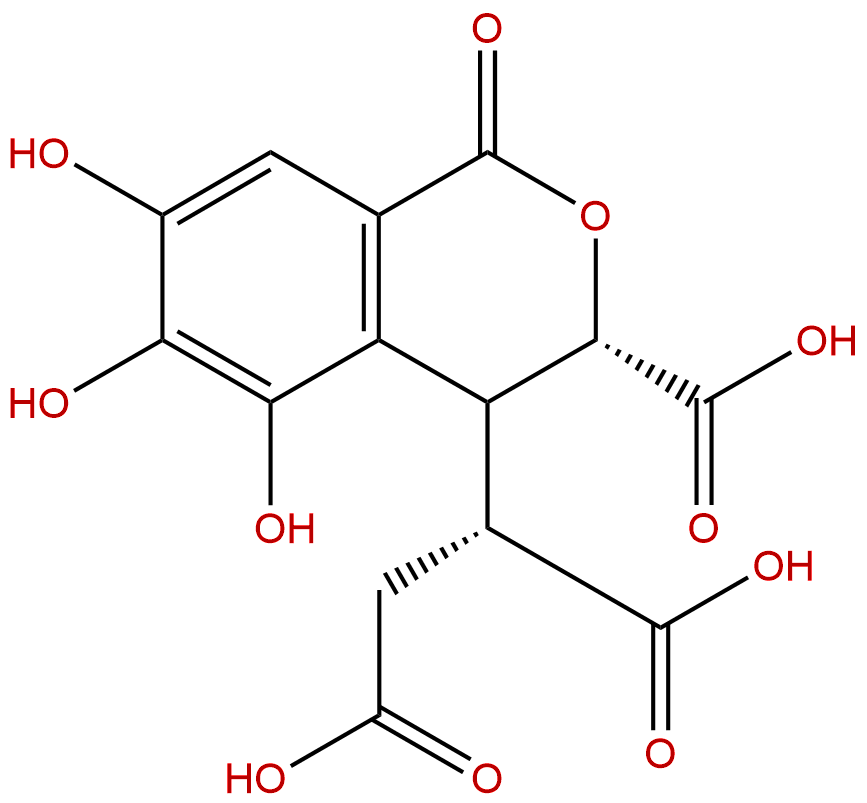
Chebulic acidCAS No.:23725-05-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP2055 |
| Formula: | C14H12O11 |
| Mol Weight: | 356.239 |
Product name: Chebulic acid
Synonym name:
Catalogue No.: BP2055
Cas No.: 23725-05-5
Formula: C14H12O11
Mol Weight: 356.239
Botanical Source: Chebulae Fructus
Physical Description:
Type of Compound: Phenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Chebulic acid has effects against the progression of AGE-induced endothelial cell dysfunction, may constitute a promising intervention agent against diabetic vascular complications; it at both doses (25 and 50 mg/kg) improves biochemical alterations caused by renal ischemia in diabetic rats. Chebulic acid significantly reduced the tert-butyl hydroperoxide (t-BHP)-induced cell cytotoxicity, intracellular reactive oxygen species level, and the ratio of GSSH, oxidized form of glutathione (GSH) to the over total GSH (GSH + GSSG) (4.42%) as compared to that with t-BHP alone (8.33%).
References:
Biol Pharm Bull. 2014;37(7):1162-7. Epub 2014 Apr 24.
Effects of chebulic acid on advanced glycation endproducts-induced collagen cross-links.
Advanced glycation end-products (AGEs) have been implicated in the development of diabetic complications.
METHODS AND RESULTS:
We report the antiglycating activity of Chebulic acid (CA), isolated from Terminalia chebula on breaking the cross-links of proteins induced by AGEs and inhibiting the formation of AGEs. Aminoguanidine (AG) reduced 50% of glycated bovine serum albumin (BSA) with glycolaldehyde (glycol-BSA)-induced cross-links of collagen at a concentration of 67.8 ± 2.5 mM, the level of CA required for exerting a similar antiglycating activity was 38.8 ± 0.5 µM. Also, the breaking activity on collagen cross-links induced by glycol-BSA was potent with CA (IC50=1.46 ± 0.05 mM), exhibiting 50-fold stronger breaking activity than with ALT-711, a well-known cross-link breaker (IC50=72.2 ± 2.4 mM). IC50 values of DPPH· scavenging activity for CA and ascorbic acid (AA) were 39.2 ± 4.9 and 19.0 ± 1.2 µg dry matter (DM) mL(-1), respectively, and ferric reducing and antioxidant power (FRAP) activities for CA and AA were 4.70 ± 0.06 and 11.4 ± 0.1 mmol/FeSO4·7H2O/g DM, respectively. The chelating activities of CA, AG and ALT711 on copper-catalyzed oxidation of AA were compared, and in increasing order, ALT-711 (IC50 of 1.92 ± 0.20 mM)<ca (ic50="" of="" 0.96="" ±="" 0.07="" mm)<ag="" (0.47="" 0.05="" mm).="" CONCLUSIONS:
Thus, CA could be a breaker as well as an inhibitor of AGE cross-linking, the activity of which may be explained in large part by its chelating and antioxidant activities, suggesting that CA may constitute a promising antiglycating candidate in intervening AGE-mediated diabetic complications.
J Ethnopharmacol. 2010 Oct 5;131(3):567-74.
Preventive effects of chebulic acid isolated from Terminalia chebula on advanced glycation endproduct-induced endothelial cell dysfunction.
The present study therefore investigated the protective mechanism of Chebulic acid, a phenolcarboxylic acid compound isolated from the ripe fruits of Terminalia chebula against advanced glycation endproducts (AGEs)-induced endothelial cell dysfunction.
METHODS AND RESULTS:
To investigate the protective mechanism of Chebulic acid against vascular endothelial dysfunction human umbilical vein endothelial cells (HUVEC) were treated with Chebulic acid in the presence/absence of glyceraldehyde-related AGEs (glycer-AGEs). HUVEC incubated with 100 μg/ml of glycer-AGEs had significantly enhanced reactive oxygen species formation, whereas the treatment of Chebulic acid dose-dependently reduced glycer-AGE-induced formation to 108.2 ± 1.9% for 25 μM versus 137.8 ± 1.1% for glycer-AGEs treated alone. The transendothelial electrical resistance (TER) value of the glycer-AGEs group was dramatically decreased to 76.9 ± 2.2% compared to the control, whereas Chebulic acid treatment prevented glycer-AGE-induced TER change with a value of 91.3 ± 5.3%. The incubation of confluent HUVEC with 100 μg/ml of glycer-AGEs for 24h remarkably increased the adhesion of human monocytic THP-1 cells compared to non-stimulated HUVEC. These increases in HUVEC adhesiveness were dose-dependently reduced by Chebulic acid.
CONCLUSIONS:
The present study shows the effects of Chebulic acid against the progression of AGE-induced endothelial cell dysfunction suggesting that this compound may constitute a promising intervention agent against diabetic vascular complications.
Arch Toxicol. 2007 Mar;81(3):211-8. Epub 2006 Aug 24.
Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes.
A hepatoprotective compound was isolated from the ethanolic extract of the fruits of Terminalia chebula Retz. by consecutive solvent partitioning, followed by silica gel and Sephadex LH-20 column chromatographies.
METHODS AND RESULTS:
The purified compound was identified as a mixture of Chebulic acid and its minor isomer, neoChebulic acid, with a ratio of 2:1 by spectroscopic analysis including 1D and 2D NMR and MS spectroscopy. To our knowledge, this is the first report on the protection of rat hepatocytes against oxidative toxicity by Chebulic acid obtained from T. chebula Retz. This compound exhibited in vitro a free radical-scavenging activity and ferric-reducing antioxidant activity. Also, the specific ESR spectrum for the (*)OOH radical signals consisting of three-line ESR spectra was within the field of 0.27 mT, whereas 2.5 and 0.25 mg/ml of Chebulic acid significantly reduced the signal intensity of the ESR spectra to 0.06 mT and 0.11 mT, respectively.
CONCLUSIONS:
Using isolated rat hepatocyte experiment, we demonstrated that the treatment of hepatocytes with Chebulic acid significantly reduced the tert-butyl hydroperoxide (t-BHP)-induced cell cytotoxicity, intracellular reactive oxygen species level, and the ratio of GSSH, oxidized form of glutathione (GSH) to the over total GSH (GSH + GSSG) (4.42%) as compared to that with t-BHP alone (8.33%).
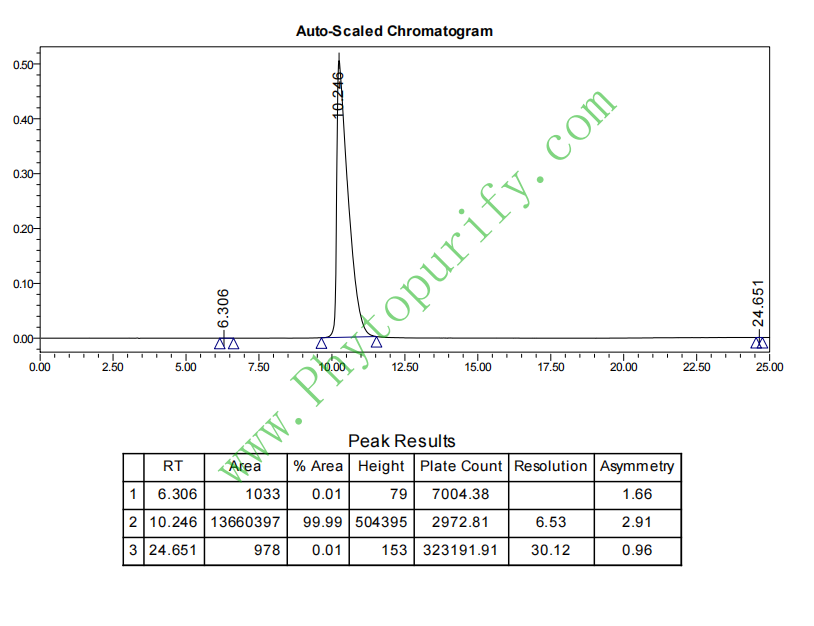
HPLC of Chebulic acid