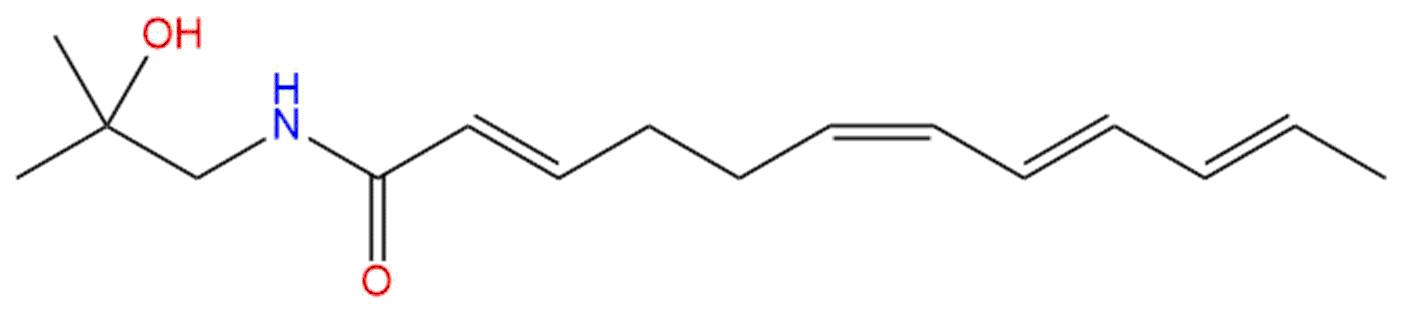
Hydroxy-α-sanshoolCAS No.:83883-10-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP4106 |
| Formula: | C16H25NO2 |
| Mol Weight: | 263.381 |
Product name: Hydroxy-α-sanshool
Synonym name:
Catalogue No.: BP4106
Cas No.: 83883-10-7
Formula: C16H25NO2
Mol Weight: 263.381
Botanical Source: Zanthoxyli pericarpium
Physical Description:
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Hydroxy-alpha-sanshool exerts antiobesity and hypolipidemic activities in HFD rats by reducing liver oxidative stress and thus could be considered as a potential candidate drug to cure or prevent obesity and hyperlipidemia. It also has analgesic properties, it activates TRPV1 and TRPA1 in sensory neurons.
References:
Biophysical Journal, 2012, 102(3):323a.
The Plant-Derived Alkylamide, Hydroxy-Alpha-Sanshool, Induces Analgesia through Inhibition of Voltage-Gated Sodium Channels
Many native cultures use extracts from Xanthozylum plants to topically treat toothache and joint pain. One active component of these extracts is the alkylamide, Hydroxy-alpha-sanshool, which induces tingling and numbing paresthesia when applied to the skin or tongue. To understand the physiological mechanisms underlying paresthesias, we sought to identify the molecular targets of sanshool in the somatosensory system.
METHODS AND RESULTS:
We first measured the analgesic properties of sanshool using mouse models of somatosensory behavior. Topical application of sanshool on the hind paw of naïve mice did not alter their sensitivity to noxious thermal or mechanical stimuli. However, in a model of neurogenic inflammation, sanshool acutely suppressed inflammatory hypersensitivity to mechanical force whereas it did not suppress hypersensitivity to heat. These data suggest that sanshool inhibits activity of a subset of sensory neurons that transduce mechanical, but not thermal, stimuli. In cultured dorsal root ganglion (DRG) neurons from mice, sanshool inhibited action potential (AP) firing in a subset of medium-to-large diameter neurons, which are thought to mediate mechanotransduction. In contrast, sanshool did not inhibit AP firing in small-diameter sensory neurons, which predominantly transduce noxious heat. In addition to size, sensory neurons are distinct in their expression of sensory neuron-specific voltage-gated sodium channels. Thus the differential effect of sanshool on sensory neurons may be due to selective activity of sanshool on different sodium channels. To test this idea, we compared the effects of sanshool on two sodium channel subtypes that are expressed in sensory neurons, Nav1.7 and 1.8. Sanshool reduced the magnitude of Nav1.7 and Nav1.8 currents but caused a hyperpolarizing shift in the steady-state inactivation curve of Nav1.7 only.
CONCLUSIONS:
Thus intrinsic molecular differences between sensory neurons, such as expression of different sodium channel subtypes, may underlie specificity of sanshool action.
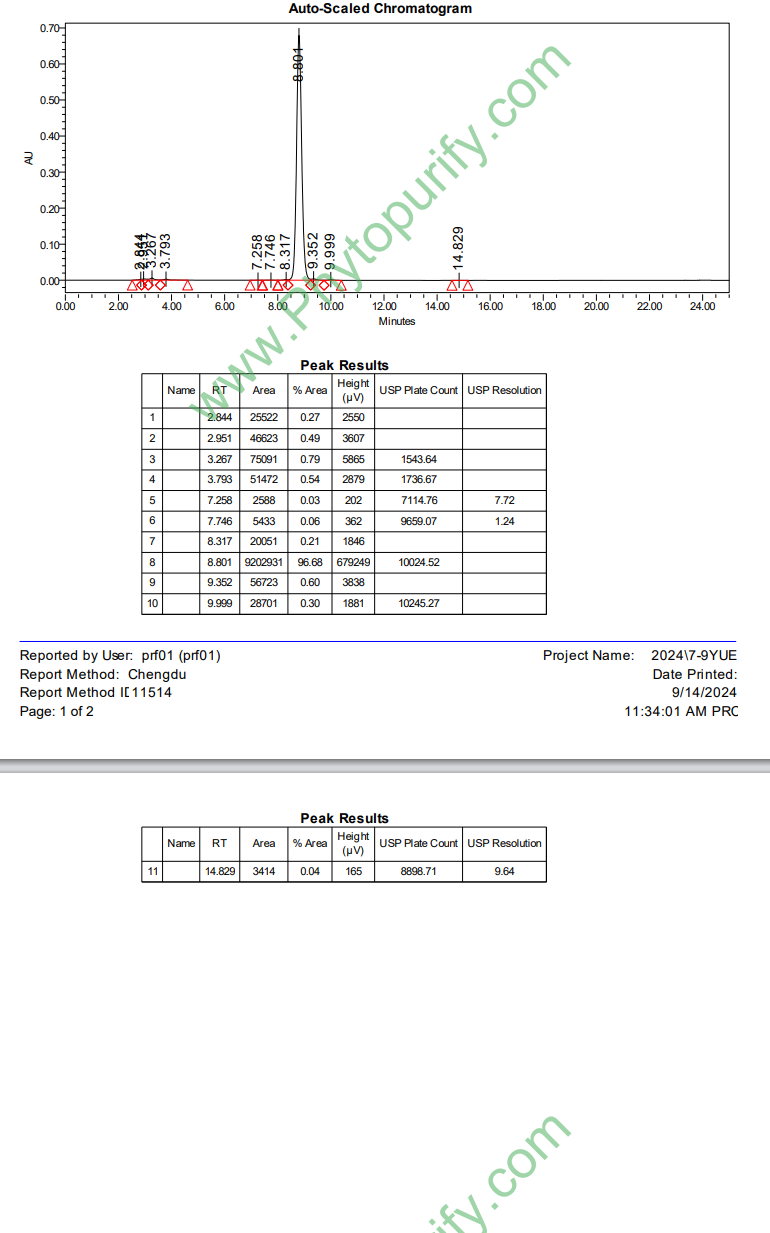
HPLC of Hydroxy-α-sanshool
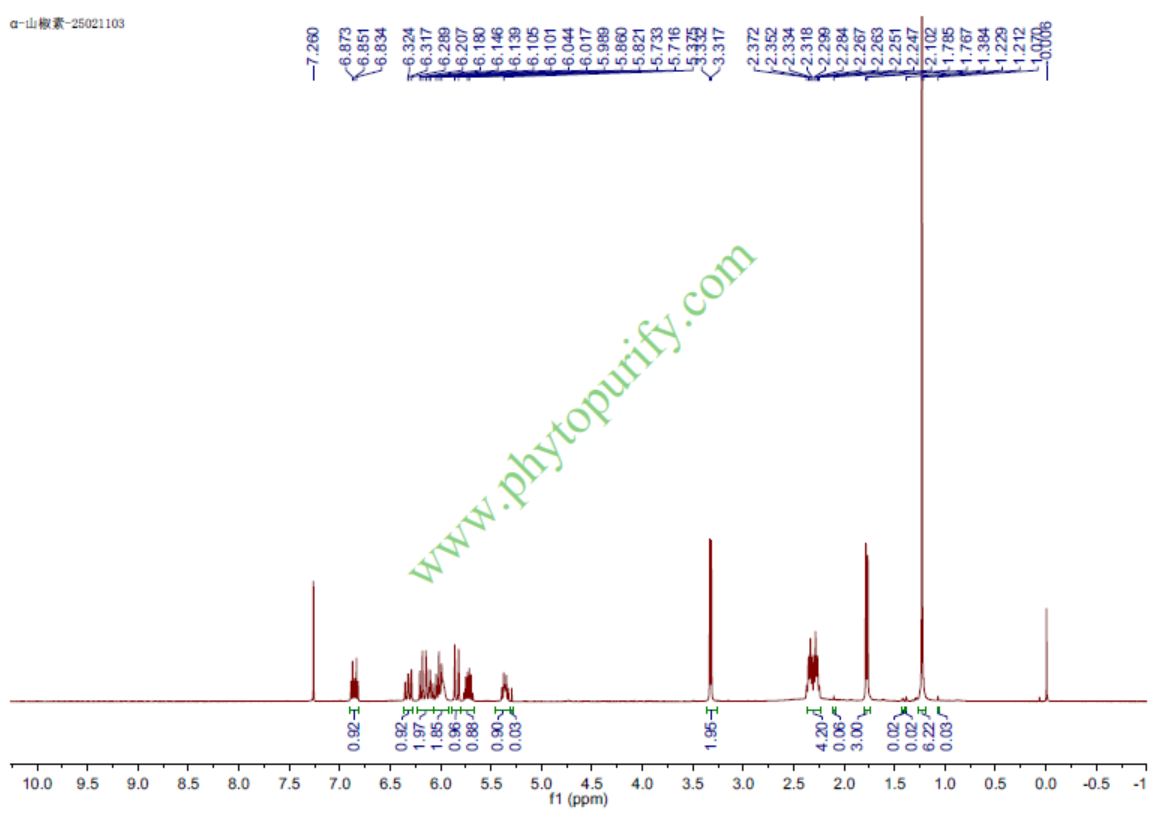
HNMR of Hydroxy-α-sanshool