
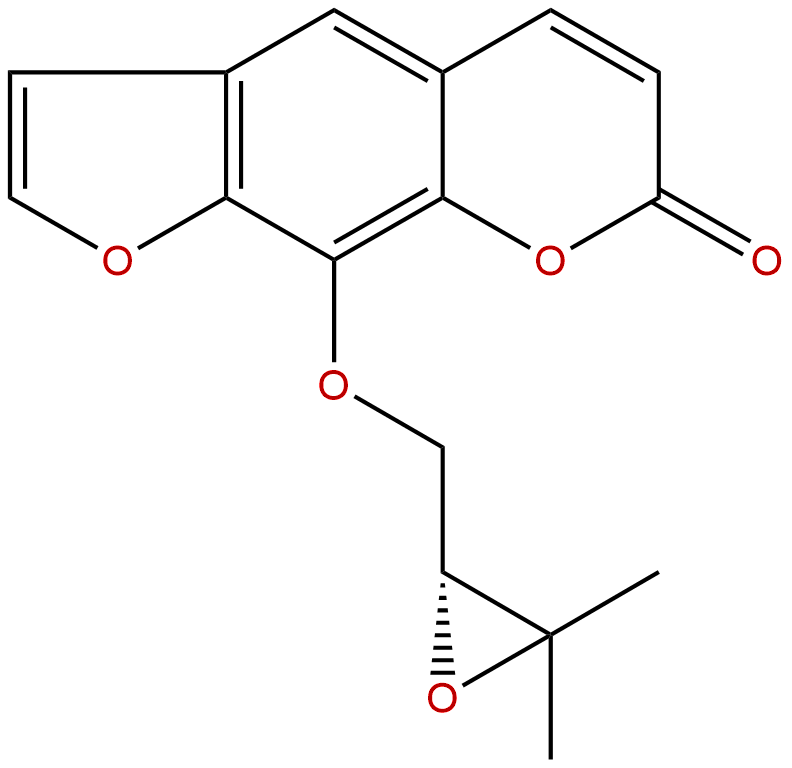
HeracleninCAS No.:2880-49-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3181 |
| Formula: | C16H14O5 |
| Mol Weight: | 286.283 |
Product name: Heraclenin
Synonym name:
Catalogue No.: BP3181
Cas No.: 2880-49-1
Formula: C16H14O5
Mol Weight: 286.283
Botanical Source:
Physical Description: Powder
Type of Compound: Coumarins
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Heraclenin has anticoagulant, and anti-inflammatory activities, it also has mutagenicity in Chlamydomonas reinhardii. Heraclenin can induce apoptosis in Jurkat leukemia cells, it has a strong clastogenic effect . (+)-Heraclenin displays significant levels of antiplasmodial and moderate levels of antimicrobial activities.
References:
Mutat Res. 1986 Jan-Feb;169(1-2):51-4.
Chromosome-damaging effects of heraclenin in human lymphocytes in vitro.
METHODS AND RESULTS:
Heraclenin, a furocoumarin with an epoxide group in its side chain, was analyzed to see if it induced structural chromosome aberrations and sister-chromatid exchanges (SCEs) in human lymphocytes in vitro. The results were compared directly with those of imperatorin, which differs from Heraclenin only in lacking an epoxide group. An equally strong clastogenic effect was found for both Heraclenin and imperatorin: the number of metaphases with breaks was increased in both cases by approximately a factor of 6. Heraclenin produced a considerable dose-dependent increase in the SCE rate, i.e., by about 60 induced SCEs/metaphase, whereas imperatorin induced only about 4 SCEs/metaphase.
CONCLUSIONS:
The results are discussed with respect to the occurrence of structural aberrations, which are primarily due to the basic furocoumarin structure itself, whereas the large increase in the SCE rate produced by Heraclenin is most probably significantly influenced by its epoxide group.
Mutat Res. 1986 Jan-Feb;169(1-2):47-50.
Mutagenicity of a furocoumarin epoxide, heraclenin, in Chlamydomonas reinhardii.
Treatment of arg- or strd mutant cells of Chlamydomonas reinhardii with a furocoumarin epoxide, Heraclenin, plus UV-A resulted in a decrease in survival and a UV-A dose-dependent increase in induced Arg+ or Strs revertants.
METHODS AND RESULTS:
Imperatorin, a furocoumarin with a very similar structure but lacking an epoxide group showed a very similar phototoxic and photomutagenic activity in these mutant strains. Treating the mutant cells with Heraclenin or imperatorin in the dark neither influenced survival nor mutation induction. The results are discussed with respect to the involvement of the epoxide moiety of Heraclenin in mutagenicity.
Curr. Sci., 2011, 100(11):1706-11.
Microbial transformation of (+)-heraclenin by Aspergillus niger and evaluation of its antiplasmodial and antimicrobial activities
METHODS AND RESULTS:
Microbial transformation of (+)-Heraclenin (1) by Aspergillus niger was studied in growth media to assess its antiplasmodial and antimicrobial activities. It was transformed to (-)-heraclenol (2) as the sole product in a stereospecific manner. The in vitro antiplasmodial activity of compounds 1 and 2 was tested with chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Further, the in vitro antibacterial activity of 1 and 2 against three Grampositive bacteria, Bacillus subtilis, Bacillus sphaericus and Staphylococcus aureus, and three Gram-negative bacteria, Pseudomonas aeruginosa, Escherichia coli and Chromobacterium violaceum was analysed using agar-plate diffusion assay. The same method was employed for the evaluation of antifungal activity against five pathogenic strains of fungi, A. niger, Rhizopus oryzae, Aspergillus flavus, Candida albicans and Saccharomyces cerevisiae.
CONCLUSIONS:
Both furanocoumarins 1 and 2 displayed significant levels of antiplasmodial and moderate levels of antimicrobial activities against the tested pathogenic strains. Compound 2 exhibited two-fold less potent antiplasmodial activity (IC50 = 6.0 μg/ml) than the parent compound 1 (IC50 = 2.5 μg/ml), whereas no difference was observed in the antimicrobial activity of both furanocoumarins. The oxirane ring was found to be beneficial in terms of antiplasmodial activity.
Planta Med. 2000 Apr;66(3):279-81.
Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model.
METHODS AND RESULTS:
From the aerial parts of Decatropis bicolor, Heraclenin (1), seselin (2), psoralen (3), imperatorin (4), skimmianine (5), and heraclenol (6), were isolated. This is the first time that coumarin-like compounds are isolated from Decatropis genus. The anti-inflammatory properties of compounds 1-6 were examined against the ear edema in mice produced by TPA.
CONCLUSIONS:
The results suggest that the anti-inflammatory activity of each compound depends of its individual substitution on the aromatic ring rather than the coumarin skeleton itself.
Indian J Physiol Pharmacol. 1969 Jul;13(3):153-5.
Anticoagulant activity of Heraclenin.
METHODS AND RESULTS:
Anticoagulant activity of Heraclenin.
HPLC of Heraclenin
