
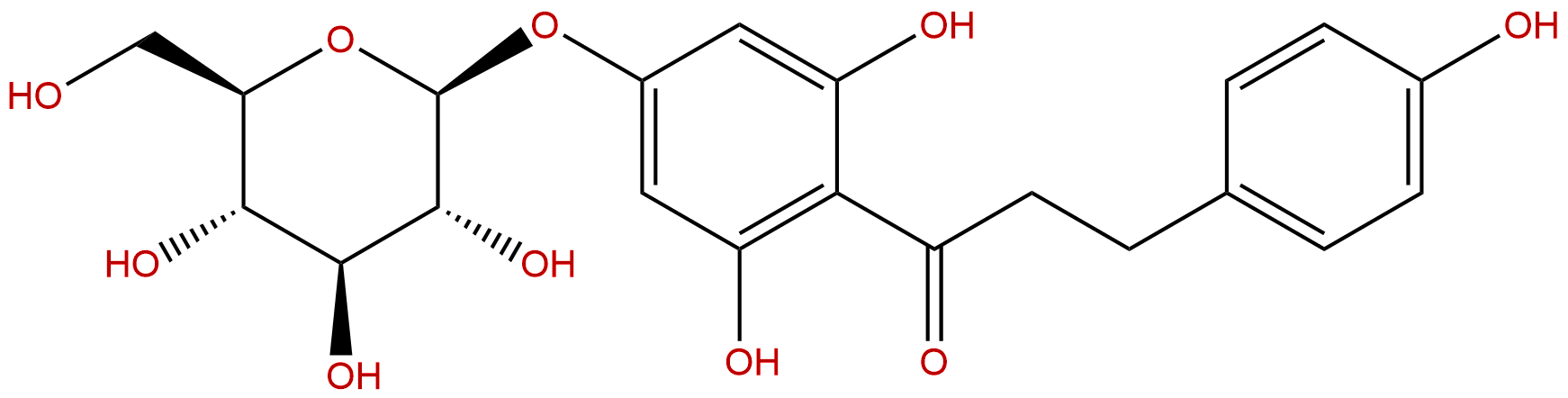
TrilobatinCAS No.:4192-90-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1756 |
| Formula: | C21H24O10 |
| Mol Weight: | 436.413 |
Product name: Trilobatin
Synonym name: p-Phloridzin
Catalogue No.: BP1756
Cas No.: 4192-90-9
Formula: C21H24O10
Mol Weight: 436.413
Botanical Source: Lithocarpus litseifolius
Physical Description: Powder
Type of Compound: Chalcones
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Trilobatin has anti-oxidant, and anti-inflammatory effects, it can increase superoxide dismutase (SOD) activity, and it potentially inhibits the lipopolysaccharide (LPS)-induced inflammatory response by suppressing the NF-κB signaling pathway. Trilobatin shows a strong inhibitory activity against α-glucosidase and a moderate inhibitory activity against α-amylase for management of postprandial hyperglycemia with less side effect.
References:
Food Chem. 2015 Jan 1;166:609-15.
Trilobatin attenuates the LPS-mediated inflammatory response by suppressing the NF-κB signaling pathway.
We investigated the anti-inflammatory effect of Trilobatin, the flavonoid isolated from the leaves of Lithocarpus polystachyus Rehd, as well as the underlying molecular mechanisms.
METHODS AND RESULTS:
Treatment with Trilobatin (0.005-5 μM) dose-dependently inhibited the lipopolysaccharide (LPS)-induced mRNA expression and secretion of pro-inflammatory cytokines, including tumor necrosis factor α (TNFα), interleukin-1β (IL-1β) and interleukin-6 (IL-6), in RAW 264.7 macrophages. However, no further inhibition was detected when the concentration of Trilobatin was increased to 50 μM. Western blot analysis confirmed that the mechanism of the anti-inflammatory effect was correlated with the inhibition of LPS-induced inhibitor of nuclear factor-kappa B α (IκBα) degradation and nuclear factor-kappa B (NF-κB) p65 phosphorylation. In addition, Trilobatin also showed a significant inhibition of LPS-induced TNFα and IL-6 at both the mRNA and protein levels in a mouse model.
CONCLUSIONS:
Our results suggest that Trilobatin potentially inhibits the LPS-induced inflammatory response by suppressing the NF-κB signaling pathway.
Z Naturforsch C. 2004 Jul-Aug;59(7-8):481-4.
Antioxidant activities of three dihydrochalcone glucosides from leaves of Lithocarpus pachyphyllus.
In vitro antioxidant activities of three sweet dihydrochalcone glucosides from the leaves of Lithocarpus pachyphyllus (Kurz) Rehd. (Fagaceae), Trilobatin 2"-acetate (1), phloridzin (2) and Trilobatin(3), were investigated.
METHODS AND RESULTS:
The IC50 (50% inhibitory concentration) values for compounds 1-3 of lipid peroxidation in rat liver homogenate were 261, 28, 88 microM, respectively. Compounds 1-3 increased superoxide dismutase (SOD) activity with EC50 (50% effective concentration) values of 575, 167, 128 microM, and glutathione peroxidase (GSH-Px) activity with EC50 values of 717, 347, 129 microM, respectively, and showed only weak DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity.