
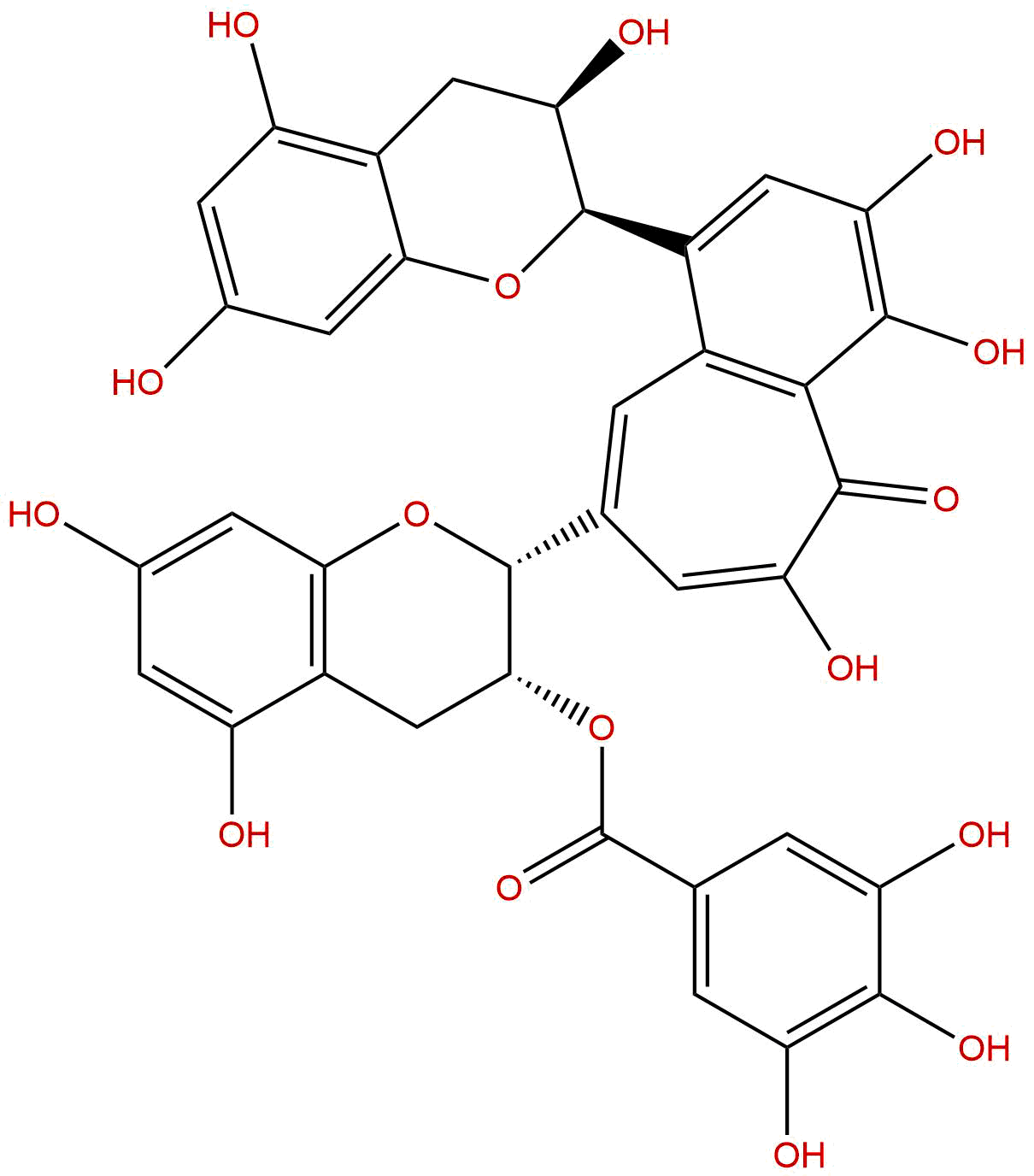
Theaflavin-3-gallateCAS No.:30462-34-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1387 |
| Formula: | C36H28O16 |
| Mol Weight: | 716.604 |
Product name: Theaflavin-3-gallate
Synonym name:
Catalogue No.: BP1387
Cas No.: 30462-34-1
Formula: C36H28O16
Mol Weight: 716.604
Botanical Source: Black Tea
Physical Description:
Type of Compound: Polyphenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Theaflavin-3-gallate has anticancer and apoptotic effects in non-small cell lung carcinoma, it acts as prooxidants and induce oxidative stress, with carcinoma cells more sensitive than normal fibroblasts. Theaflavin-3-gallate can play a role in decreased intestinal cholesterol absorption via inhibition of micelle formation.
References:
Basic Clin Pharmacol Toxicol. 2008 Jul;103(1):66-74.
Theaflavin-3-gallate and theaflavin-3'-gallate, polyphenols in black tea with prooxidant properties.
This study compared the in vitro responses of human gingival fibroblasts and of carcinoma cells derived from the tongue to Theaflavin-3-gallate (TF-2A) and theaflavin-3'-gallate (TF-2B), polyphenols in black tea.
METHODS AND RESULTS:
The antiproliferative and cytotoxic effects of the theaflavin monomers were more pronounced to the carcinoma, than to the normal, cells. In phosphate buffer at pH 7.4, the theaflavins generated hydrogen peroxide and the superoxide anion, suggesting that their mode of toxicity may be due, in part, to the induction of oxidative stress. In a cell-free assay, Theaflavin-3-gallate and TF-2B reacted directly with reduced glutathione (GSH), in a time- and concentration-dependent manner. Intracellular storages of GSH were depleted on treatment of the cells with the theaflavin monomers. Depletion of intracellular GSH was more extensive with Theaflavin-3-gallate than with TF-2B and was more pronounced in the carcinoma, than in the normal, cells. The toxicities of the theaflavins were potentiated when the cells were cotreated with the GSH depleter, d,l-buthionine-[S,R]-sulfoximine. In the presence of catalase, pyruvate and divalent cobalt, all scavengers of reactive oxygen species, the cytotoxicities of the theaflavins were lessened. Theaflavin-3-gallate and TF-2B induced lipid peroxidation in the carcinoma cells, whereas in the fibroblasts, peroxidation was evident upon exposure to Theaflavin-3-gallate, but not to TF-2B.
CONCLUSIONS:
These studies demonstrated that the black tea theaflavin monomers, Theaflavin-3-gallate and TF-2B, act as prooxidants and induce oxidative stress, with carcinoma cells more sensitive than normal fibroblasts.
Bangl. J. Pharmacol., 2015, 10(4):3047-53.
Anticancer and apoptotic effects of theaflavin-3-gallate in non-small cell lung carcinoma.
The objective was to determine the antiproliferative and apoptotic effects of Theaflavin-3-gallate in human non-small cell lung cancer cells (A-549) along with determining the effect on cell cycle phase distribution, cell migration and invasion.
METHODS AND RESULTS:
Cell viability was determined by MTT assay while as phase contrast and fluorescence microscopies were involved to study apoptotic morphologi-cal features in these cells. Flow cytometry investigated the effect of Theaflavin-3-gallate on cell cycle phase distribution. Theaflavin-3-gallate treatment led to a substantial cytotoxic effect in A-549 cancer cells with IC50 values of 42.1 μM and 27.9 μM at 24 and 48 hours respectively. Further, 80 and 160 μM dose of Theaflavin-3-gallate induced apoptotic features including chromatin margina-tion and micronuclei presence. The population of cells in G2/M phase increased from 2.7% (control) to 6.8%, 17.2% and finally to 46.5% after treatment with 20, 80 and 160 μM concentration of Theaflavin-3-gallate respectively indicating G2/M phase cell cycle arrest.
HPLC of Theaflavin-3-gallate
