
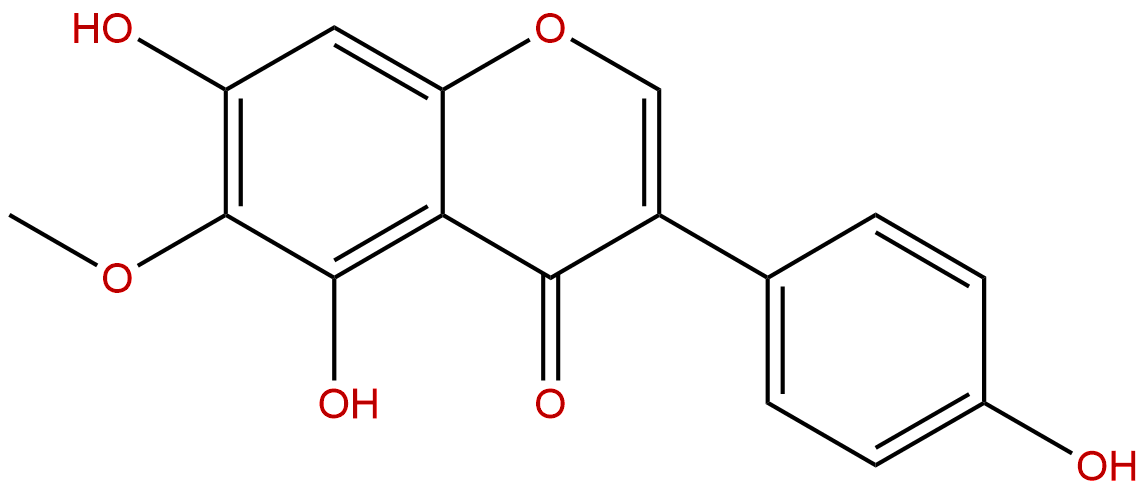
TectorigeninCAS No.:548-77-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1369 |
| Formula: | C16H12O6 |
| Mol Weight: | 300.266 |
Product name: Tectorigenin
Synonym name:
Catalogue No.: BP1369
Cas No.: 548-77-6
Formula: C16H12O6
Mol Weight: 300.266
Botanical Source: Baptisia spp., Centrosema spp., Dalbergia spp. and Ononis spinosa (all Leguminosae, Papilionoideae) and also Iris germanica
Physical Description: Yellow cryst.
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Tectorigenin (Tg) and tectoridin (Td) are the major compounds isolated from the rhizomes of iridaceous plant Belamcanda chinensis which is well known as a chinese traditional medicine for the treatment of inflammatory diseases; Tectorigenin inhibits IFN-γ/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells, it appears to have the potential to prevent inflammation.[1]
Tectorigenin and several other phytochemicals downregulate PDEF, PSA and IGF-1 receptor mRNA expression in vitro, the downregulation of PDEF, PSA, hTERT and IGF-1 receptor gene expression by tectorigenin demonstrates the antiproliferative potential of these agents, they might be new and established targets for therapies in prostate cancer.[2]
Tectorigenin and kaikasaponin III have hypoglycemic and hypolipidemic effects in the streptozotocin-induced diabetic rat and their antioxidant activity.[3]
Tectorigenin-paclitaxel-induces nuclear translocation of NFκB and the phosphorylation of IκB and IKK, suggests that tectorigenin could sensitize paclitaxel-resistant human ovarian cancer cells through inactivation of the Akt/IKK/IκB/NFκB signaling pathway, and promise a new intervention to chemosensitize paclitaxel-induced cytotoxicity in ovarian cancer.[4]
Tectorigenin has inhibitory effect of the activities of plasma ALT, the effects is much more potent than that of a commercially available dimethyl diphenyl bicarboxylate; Orally administered tectoridin shows hepatoprotective activity; tectorigenin also protects against the cytotoxicity of HepG2 cells induced by t-BHP, this protection may have originated from the inhibition of apoptosis; tectorigenin may be hepatoprotective and tectoridin should be a prodrug that is transformed to tectorigenin.[5]
Tectorigenin inhibits the in vitro proliferation and enhances miR-338* expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis.[6]
References:
[1] Pan C H, Kim E S, Sang H J, et al. Arch Pharm Res, 2008, 31(11):1447-56.
[2] Thelen P, Scharf J G, Burfeind P, et al. Carcinogenesis, 2005, 26(8):1360-7.
[3] Lee K T, Sohn I C, Dong H K, et al. Arch Pharm Res, 2000, 23(5):461-6.
[4] Genead R, Fischer H, Hussain A, et al. Carcinogenesis, 2012, 33(12):2488-98.
[5] Lee H U, Bae E A, Kim D H. J Pharmacol Sci, 2005, 97(97):541-4.
[6] Zhang H, Liu X, Shi C, et al. J Ethnopharmacol, 2010, 131(1):165-73.
[7] Zhao N, Liu D, Wang Y P, et al. J Pharm Practice, 2012, 3.
HPLC of Tectorigenin
