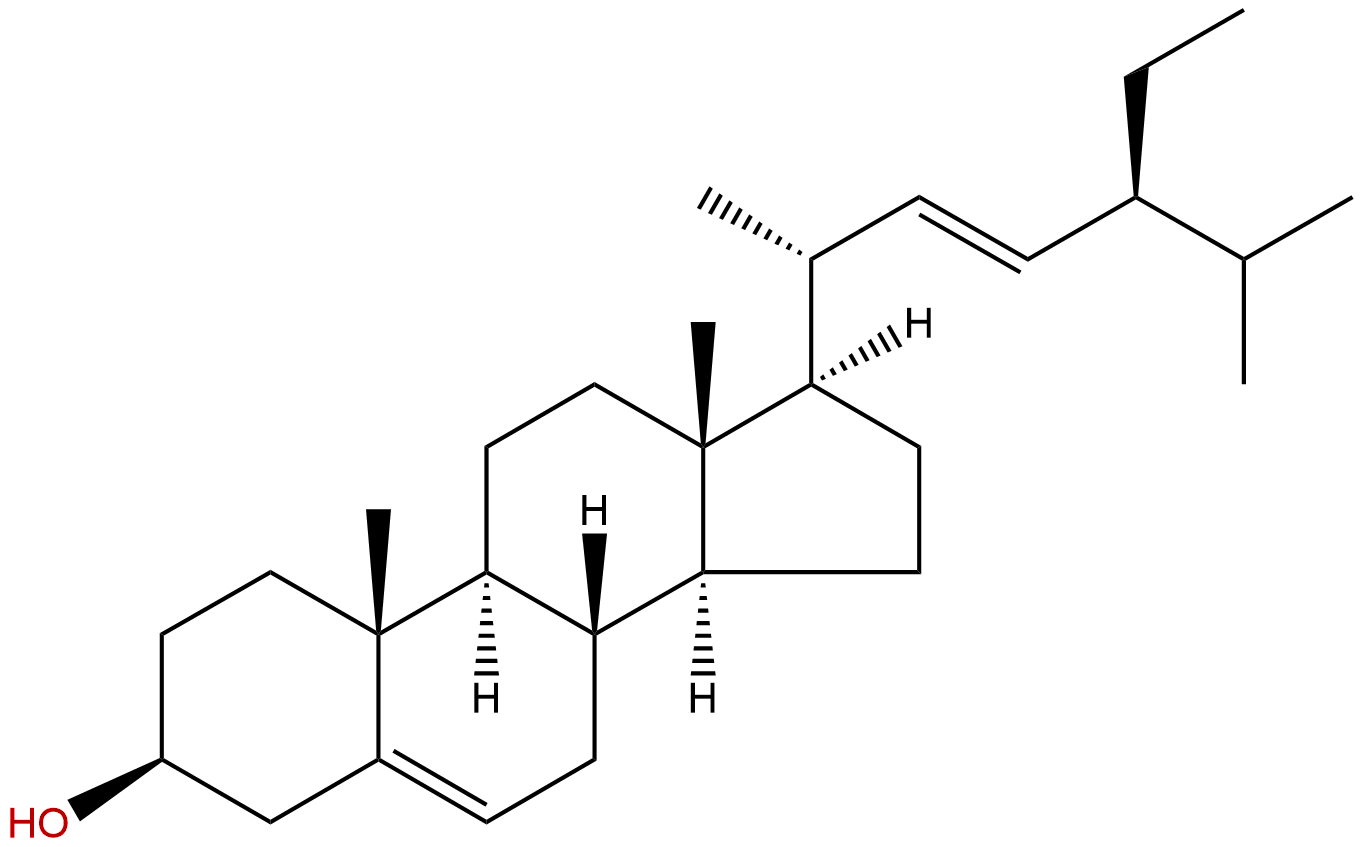
StigmasterolCAS No.:83-48-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1343 |
| Formula: | C29H48O |
| Mol Weight: | 412.702 |
Product name: Stigmasterol
Synonym name: Serposterol
Catalogue No.: BP1343
Cas No.: 83-48-7
Formula: C29H48O
Mol Weight: 412.702
Botanical Source: Widely occurring sterol. Present in potato (Solanum tuberosum), chick pea (Cicer arietinum), artichoke (Cynara scolymus), sunflower oil, olive oil, beans (Phaseolus vulgaris), oats and strawberry fruit. Also widely distributed in marine organisms such as
Physical Description: Powder
Type of Compound: Steroids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR.[1]
Stigmasterol, isolated from the bark of Butea monosperma, has thyroid inhibitory, anti-peroxidative and hypoglycemic effects .[2]
Stigmasterol has potential anti-osteoarthritic properties, can inhibit several pro-inflammatory and matrix degradation mediators typically involved in OA-induced cartilage degradation, at least in part through the inhibition of the NF-kappaB pathway.[3]
Stigmasterol can inhibit tumour promotion in mouse skin two-stage carcinogenesis.[4]
Stigmasterol can induce cognitive ameliorative effects, mediated by the enhancement of cholinergic neurotransmission system via the activation of estrogen or NMDA receptors.[5]
Stigmasterol, when fed, lowers plasma cholesterol levels, inhibits intestinal cholesterol and plant sterol absorption, and suppresses hepatic cholesterol and classic bile acid synthesis in Wistar as well as WKY rats. However, plasma and hepatic incorporation of stigmasterol is low.[6]
References:
[1] Carter B A, Taylor O A, Prendergast D R, et al. Pediatric Res, 2007, 62(3):301-6.
[2] Panda S, Jafri M, Kar A, et al. Fitoterapia, 2009, 80(2):123-6.
[3] Gabay O, Sanchez C, Salvat C, et al. Osteoarthr Cartilage, 2010, 18(1):106-16.
[4] Kasahara Y, Kumaki K, Katagiri S, et al. Phytother Res 1994, 8(6):327-31.
[5] Park S J, Dong H K, Jung J M, et al. Eur J Pharmacol, 2012, 676(1-3):64-70.
[6] Batta A K, Xu G, Honda A, et al. Metabolism , 2006, 55(3):292-9.
[7] Liu S Y, Sun L J, Guo X. Adv Materials Res, 2011, 233-235:1206-9.
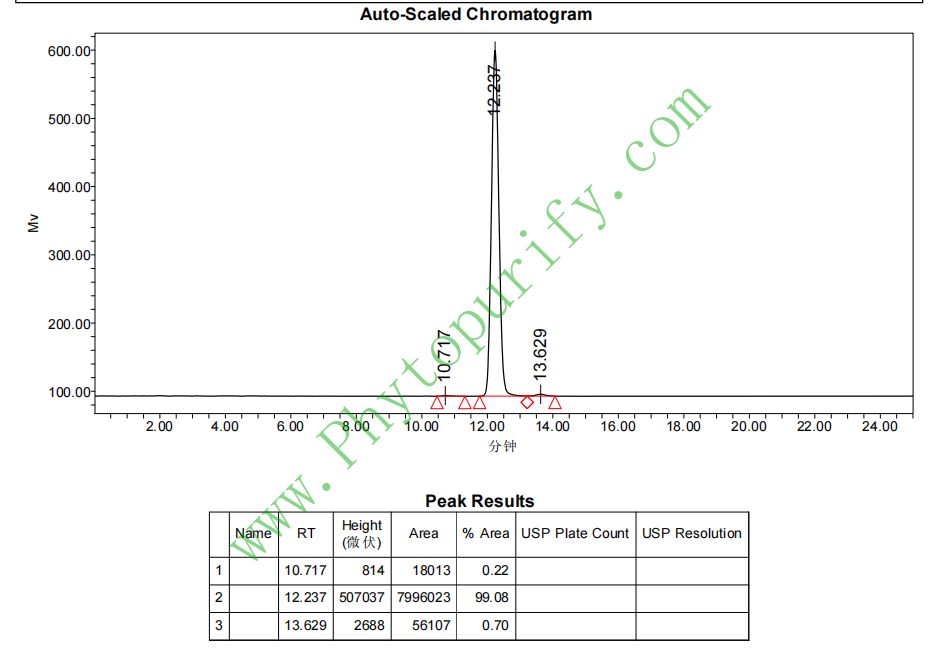
HPLC of Stigmasterol
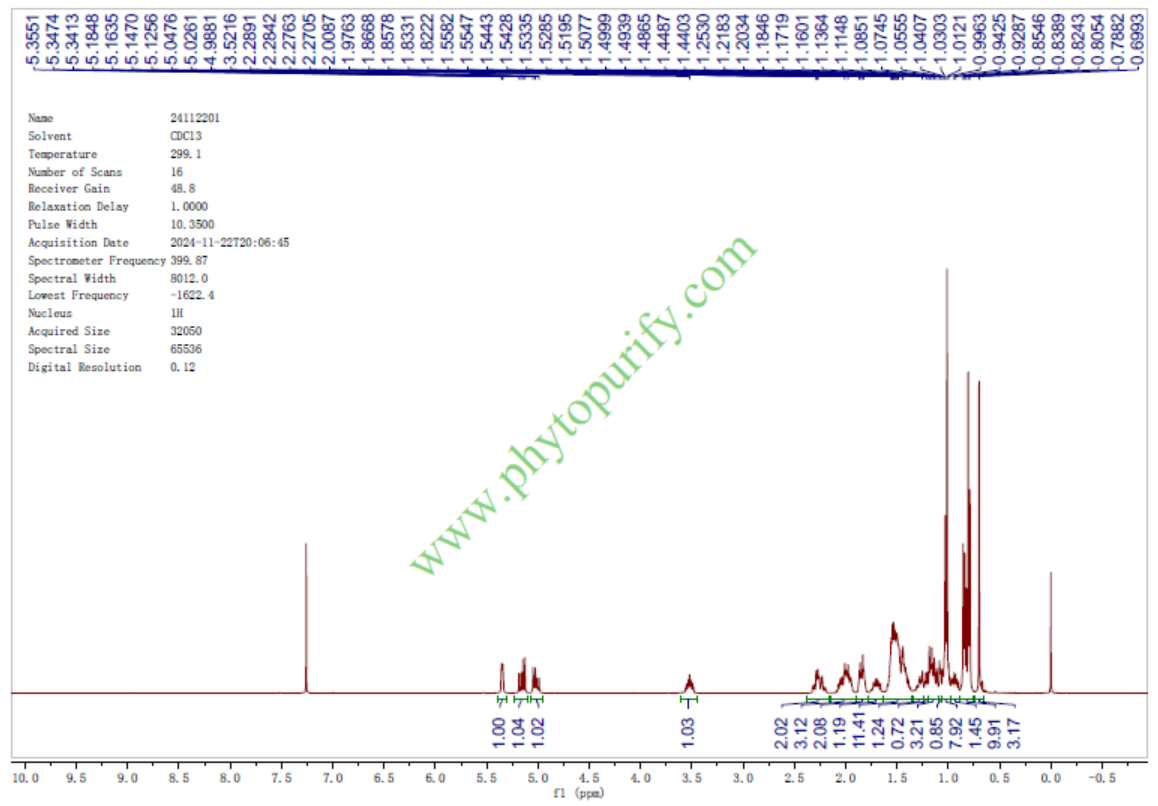
HNMR of Stigmasterol