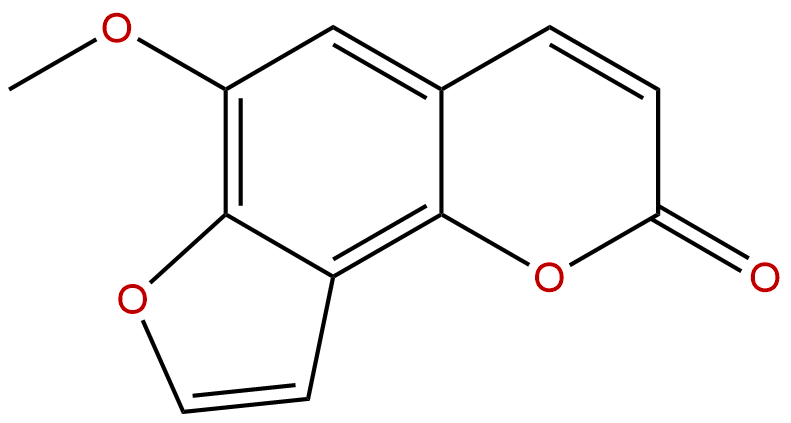
Sphondin Descrtption
Product name: Sphondin
Synonym name: 6-Methoxyangelicin
Catalogue No.: BP1335
Cas No.: 483-66-9
Formula: C12H8O4
Mol Weight: 216.192
Botanical Source: From seeds of Heracleum sphondylium, Heracleum lanatum other Heracleum spp., Ruta pinnata and Pimpinella saxifraga
Physical Description: Powder
Type of Compound: Coumarins
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Sphondin, a furanocoumarin derivative isolated from Heracleum laciniatum, possesses an inhibitory effect on IL-1beta-induced increase in the level of COX-2 protein and PGE(2) release in A549 cells, the inhibitory mechanism, at least in part, through suppression of NF-kappaB activity, suggests that sphondin may have the therapeutic potential as an anti-inflammatory drug on airway inflammation.[1]
Sphondin, 8-methoxypsoralen, and khellin have delayed phototoxic effects inAedes aegypti.[2]
Sphondin has NO production inhibitory activity, due to the effect of iNOS expression, but not by direct inhibition of iNOS enzyme activity, thus, sphondin may act as a potent inhibitor of NO production under tissue-damaging inflammatory conditions.[3]
Sphondin, one of furanocoumarins isolated from the fruits of the plant, may have anticonvulsant activity.[4]
Sphondin shows anti-proliferative activity and causes G2/M arrest at concentrations of 0.05-15.0 uM, it may have anti-tumor effects.[5]
References:
[1] Yang L L, Liang Y C, Chang C W, et al. Life Sci, 2002, 72(2):199-213.
[2] Kagan J, Szczepanski P, Bindokas V, et al. J Chem Ecol, 1986, 12(4):899-914.
[3] Wang C C, Lai J E, Chen L G, et al. Bioorg Med Chem, 2000, 8(12):2701-7.
[4] Tosun F, ?i?dem Akyüz K?z?lay, Erol K, et al. Food Chem, 2008, 107(3):990-3.
[5] Sumiyoshi M, Sakanaka M, Taniguchi M, et al. J Nat Med, 2013, 68(1):83-94.
[6] Fatma M. Al-Barwani, Elsadig A. Eltayeb. SQU Journal For Science, 2004,(9): 7-17.