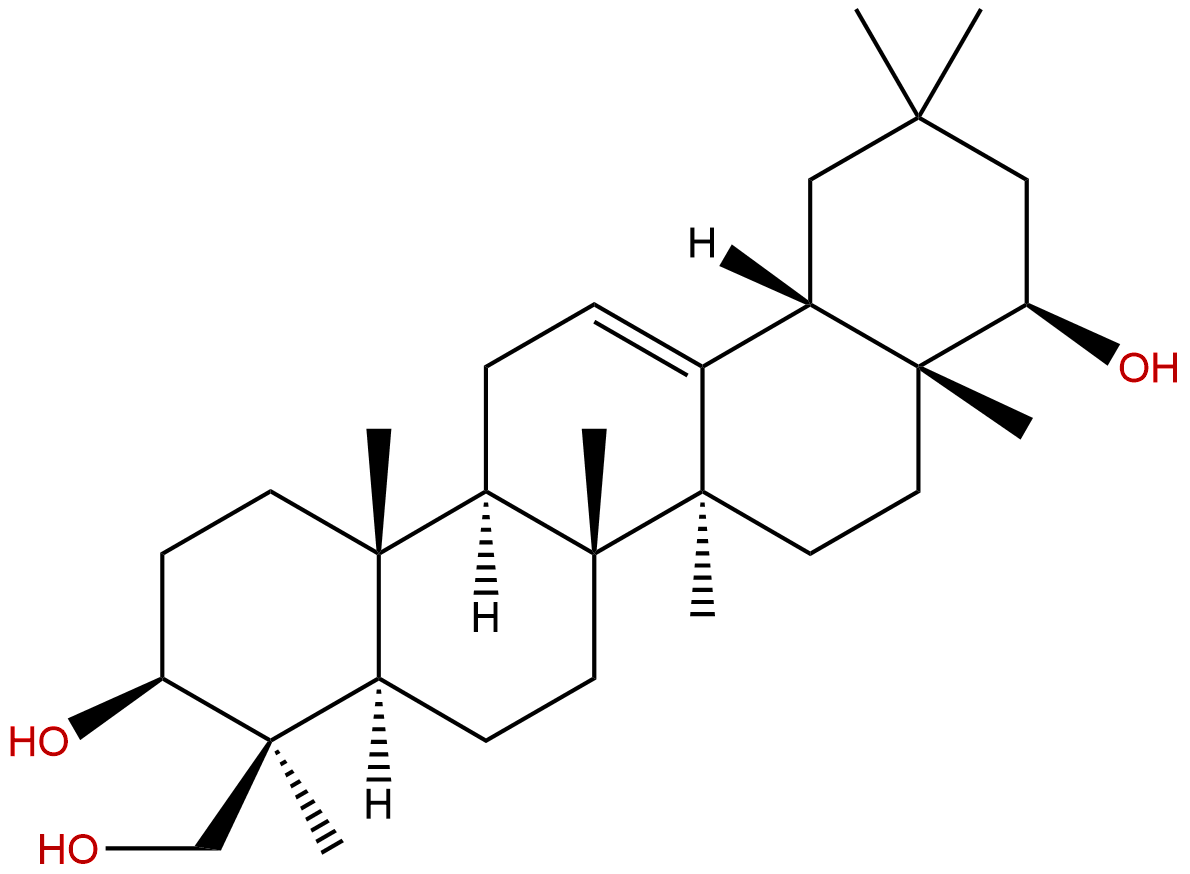
Soyasapogenol BCAS No.:595-15-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1329 |
| Formula: | C30H50O3 |
| Mol Weight: | 458.727 |
Synonym name: Soyasapogenol M2
Catalogue No.: BP1329
Cas No.: 595-15-3
Formula: C30H50O3
Mol Weight: 458.727
Botanical Source: soya bean saponin, Medicago, Astragalus, Trifolium spp. and Streptomyces sp.
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Soyasapogenol B has anti-cancer, hypocholesterolemic, anticomplementary, hepatoprotective effects, it inhibits proliferation of cultured Hep-G2.
References:
Biol Pharm Bull. 2003 Sep;26(9):1357-60.
Hepatoprotective constituents in plants. 14. Effects of soyasapogenol B, sophoradiol, and their glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells.
The effects of Soyasapogenol B, sophoradiol, their glucuronides, and glycyrrhizin on the hepatotoxicity of tert-butyl hydroperoxide (t-BuOOH) in a human-liver-derived cell line (HepG2 cells) were investigated.
METHODS AND RESULTS:
Glycyrrhizin showed significant dose-dependent protective effects against the cytotoxicity of t-BuOOH. Among Soyasapogenol B and its glucuronides, the monoglucuronide showed the most potent hepatoprotective activity, followed by Soyasapogenol B itself. Soyasaponin III was weakly protective, while soyasaponin I increased the toxicity of t-BuOOH. Among sophoradiol and its glucuronides, sophoradiol itself showed the most potent hepatoprotective activity, which was equal to glycyrrhizin, while the monoglucuronide and kaikasaponin III showed an increase in cytotoxicity. These results were considerably different from those reported previously on the protective effects of these compounds using primary cultures of immunologically injured rat liver cells.
CONCLUSIONS:
Consequently, the hepatoprotective action of the triterpene derivatives investigated would be different in HepG2 cells and in rat primary hepatocyte cultures.
J Agric Food Chem. 2008 Apr 23;56(8):2603-8.
Effect of soyasapogenol A and soyasapogenol B concentrated extracts on HEP-G2 cell proliferation and apoptosis.
The growth inhibition and the induction of apoptosis brought about by soyasaponins extracted from soy flour ( Glycine max (L.)) and concentrated for soyasapogenols A and B formed by hydrolysis were tested for cytoactivity in the human hepatocellular carcinoma cell line Hep-G2.
METHODS AND RESULTS:
Concentrated soyasapogenol A (SG-A) and Soyasapogenol B (SG-B) extracts contained approximately 69.3% and 46.2% of their respective aglycones (soyasapogenols) assessed by HPLC and ESI-MS, while the soyasaponin extract (TS), derived from crude methanol extraction, did not contain any detectable amounts of SG-A or SG-B. An MTT viability assay showed that all three extracts had an effect on Hep-G2 proliferation in a dose-response manner with 72 h LC50 values of 0.594+/-0.021 mg/mL for TS, 0.052+/-0.011 mg/mL for SG-A, and 0.128+/-0.005 mg/mL for SG-B. Apoptotic cells were determined by flow cytometry cell cycle analysis and confocal laser scanning microscopy (CLSM). Cell cycle analysis indicated a significant ( P< 0.05) greater sub-G1 buildup of apoptotic cells at 24 h (25.63+/-2.1%) and 72 h (47.1+/-3.5%) for the SG-A extract compared to SG-B, whereas the TS extract produced only a minor buildup of sub-G1 cells. CLSM confirmed a morphological change of all treatments after 24 h, at the respective LC50 concentrations.
CONCLUSIONS:
These results show that the samples that contained mainly soyasapogenols A and B showed a greater ability to inhibit proliferation of cultured Hep-G2 when compared to a total soyasaponin extract that did not contain any soyasapogenols.
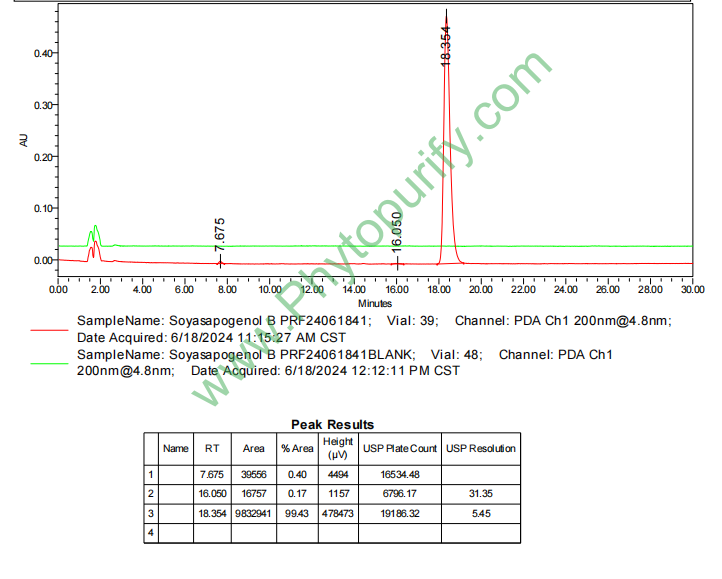
HPLC of Soyasapogenol B