
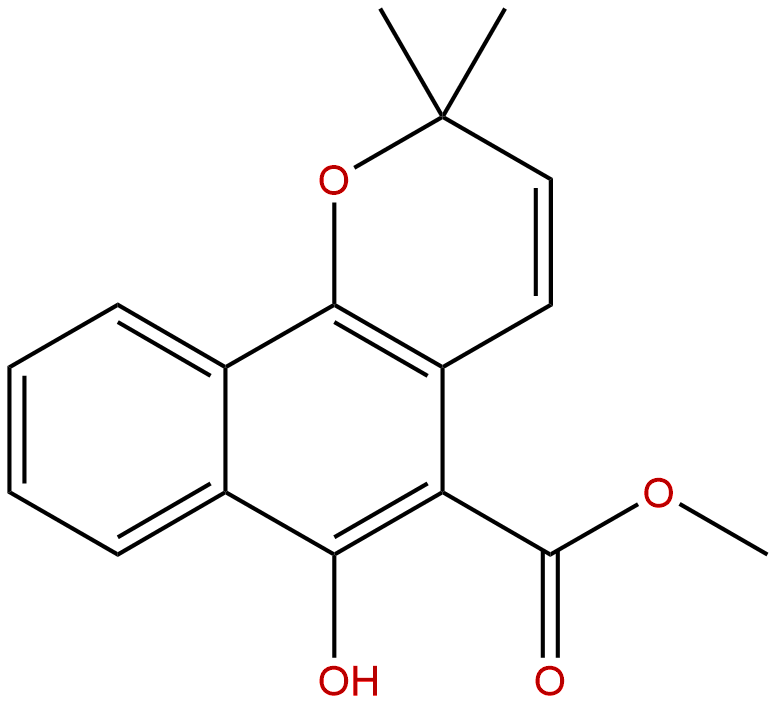
MolluginCAS No.:55481-88-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1228 |
| Formula: | C17H16O4 |
| Mol Weight: | 284.311 |
Product name: Mollugin
Synonym name: Rubimaillin
Catalogue No.: BP1228
Cas No.: 55481-88-4
Formula: C17H16O4
Mol Weight: 284.311
Botanical Source: Rubia cordifolia L.
Physical Description:
Type of Compound: Sesquiterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Mollugin is a JAK2 inhibitor and inhibits LPS-induced inflammatory responses by blocking the activation of the JAK-STAT pathway. Mollugin may be a novel therapeutic candidate for bone loss-associated disorders including osteoporosis, rheumatoid arthritis, and periodontitis. Mollugin has anticancer efficacy, can modulate the HER2, HO-1, Nrf2 , and NF- κB pathways.
References:
Biochem Biophys Res Commun. 2014 Jul 18;450(1):247-54.
Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways.
Mollugin, a bioactive phytochemical isolated from Rubia cordifolia L., has shown preclinical anticancer efficacy in various cancer models. However the effects of Mollugin in regulating cancer cell survival and death remains undefined.
METHODS AND RESULTS:
In the present study we found that Mollugin exhibited cytotoxicity on various cancer models. The suppression of cell viability was due to the induction of mitochondria apoptosis. In addition, the presence of autophagic hallmarks was observed in Mollugin-treated cells. Notably, blockade of autophagy by a chemical inhibitor or RNA interference enhanced the cytotoxicity of Mollugin. Further experiments demonstrated that phosphatidylinositide 3-kinases/protein kinase B/mammalian target of rapamycin/p70S6 kinase (PI3K/AKT/mTOR/p70S6K) and extracellular regulated protein kinases (ERK) signaling pathways participated in Mollugin-induced autophagy and apoptosis.
CONCLUSIONS:
Together, these findings support further studies of Mollugin as candidate for treatment of human cancer cells.
Phytomedicine. 2015 Jan 15;22(1):27-35.
Mollugin from Rubea cordifolia suppresses receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis and bone resorbing activity in vitro and prevents lipopolysaccharide-induced bone loss in vivo.
Osteopenic diseases, such as osteoporosis, are characterized by progressive and excessive bone resorption mediated by enhanced receptor activator of nuclear factor-κB ligand (RANKL) signaling. Therefore, downregulation of RANKL downstream signals may be a valuable approach for the treatment of bone loss-associated disorders.
METHODS AND RESULTS:
In this study, we investigated the effects of the naphthohydroquinone Mollugin on osteoclastogenesis and its function in vitro and in vivo. Mollugin efficiently suppressed RANKL-induced osteoclast differentiation of bone marrow macrophages (BMMs) and bone resorbing activity of mature osteoclasts by inhibiting RANKL-induced c-Fos and NFATc1 expression. Mollugin reduced the phosphorylation of signaling pathways activated in the early stages of osteoclast differentiation, including the MAP kinase, Akt, and GSK3β and inhibited the expression of different genes associated with osteoclastogenesis, such as OSCAR, TRAP, DC-STAMP, OC-STAMP, integrin αν, integrin β3, cathepsin K, and ICAM-1. Furthermore, mice treated with Mollugin showed significant restoration of lipopolysaccharide (LPS)-induced bone loss as indicated by micro-CT and histological analysis of femurs.
CONCLUSIONS:
Consequently, these results suggested that Mollugin could be a novel therapeutic candidate for bone loss-associated disorders including osteoporosis, rheumatoid arthritis, and periodontitis.