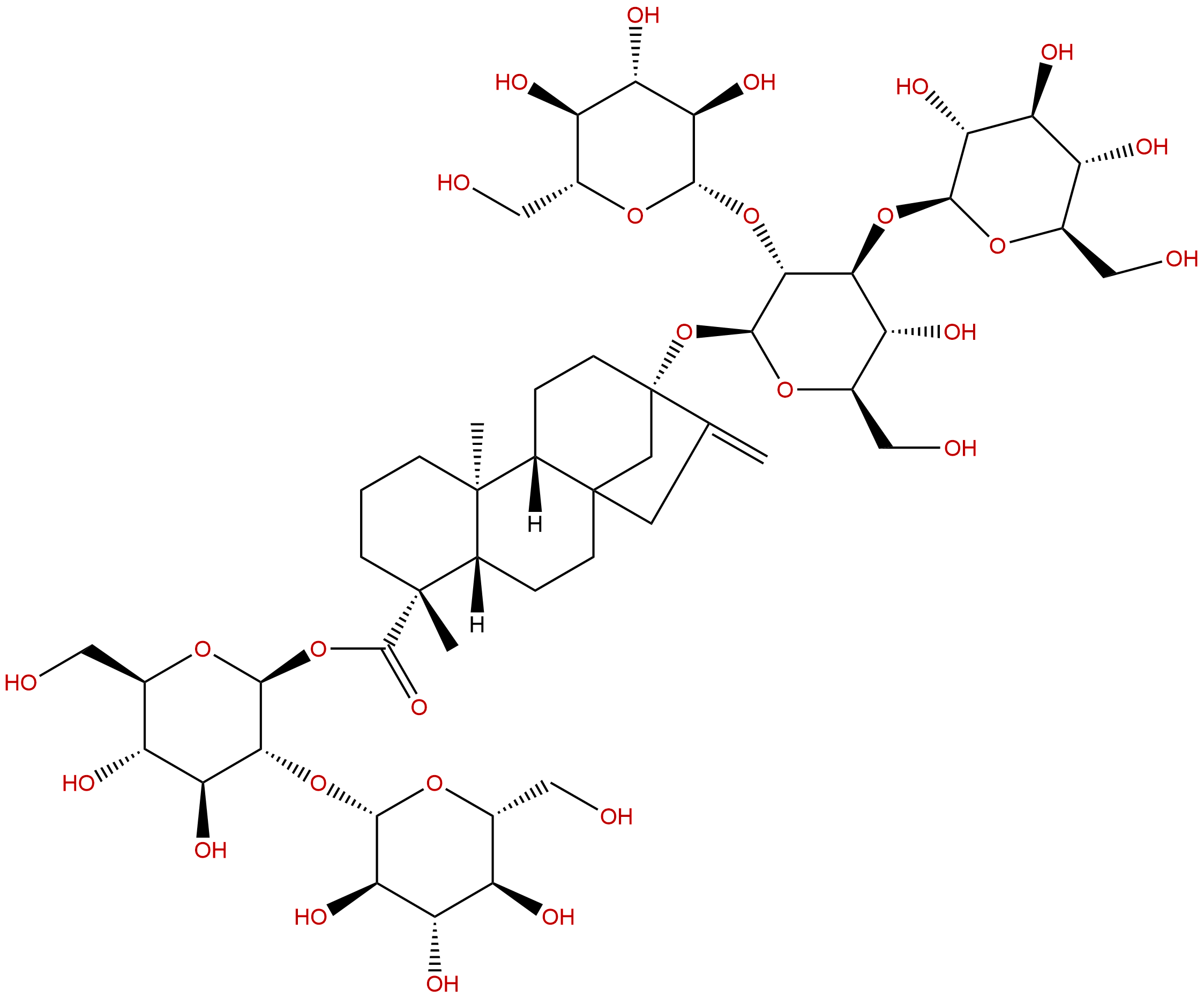
Rebaudioside DCAS No.:63279-13-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1200 |
| Formula: | C50H80O28 |
| Mol Weight: | 1129.16 |
Product name: Rebaudioside D
Synonym name:
Catalogue No.: BP1200
Cas No.: 63279-13-0
Formula: C50H80O28
Mol Weight: 1129.16
Botanical Source: Stevia rebaudiana (Bertoni) Hemsl.
Physical Description:
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Rebaudioside D is a potential sweetener. Rebaudioside D shows similar stability when exposed to simulate stomach and small intestine fluids, with susceptibility to hydrolytic degradation by enteric bacteria collected from the cecum.
References:
Int J Toxicol. 2013 Jul;32(4):261-73.
Metabolism and toxicity studies supporting the safety of rebaudioside D.
Rebaudioside D (Reb D) is one of the several glycosides found in the leaves of Stevia rebaudiana (Bertoni) Bertoni (Compositae) which has been identified as a potential sweetener.
METHODS AND RESULTS:
The metabolism of Reb A and Rebaudioside D was evaluated in various in vitro matrices (simulated gastrointestinal fluids, rat liver microsomes, and rat cecal contents) and through analysis of plasma collected from rats in a dietary toxicity study. Reb A and Rebaudioside D showed similar stability when exposed to simulated stomach and small intestine fluids, with susceptibility to hydrolytic degradation by enteric bacteria collected from the cecum. Incubations with rat liver microsomes indicated that neither compound is expected to be metabolized by the liver enzymes. Plasma concentrations of Rebaudioside D, Reb A, and/or the final hydrolysis product of each compound, free/conjugated steviol, were consistent between animals administered either Rebaudioside D or Reb A in the diet. A repeated exposure dietary toxicity study was conducted to compare the safety of Rebaudioside D, when administered at target exposure levels of 500, 1000, and 2000 mg/kg body weight (bw)/d to Sprague-Dawley rats for 28 days, to that of Reb A administered at a target exposure level of 2000 mg/kg bw/d. There were no treatment-related effects on the general condition and behavior of the animals and no toxicologically relevant, treatment-related effects on hematology, serum chemistry, or urinalysis.
CONCLUSIONS:
Macroscopic and microscopic findings revealed no treatment-related effects on any organ evaluated. Results were comparable between the group administered 2000 mg/kg/d Rebaudioside D and the group administered 2000 mg/kg/d Reb A.
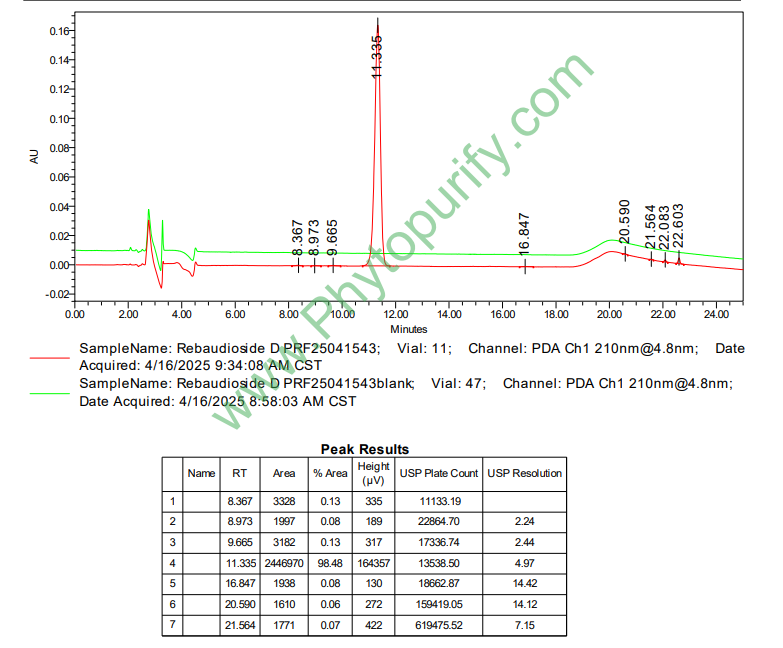
HPLC of Rebaudioside D