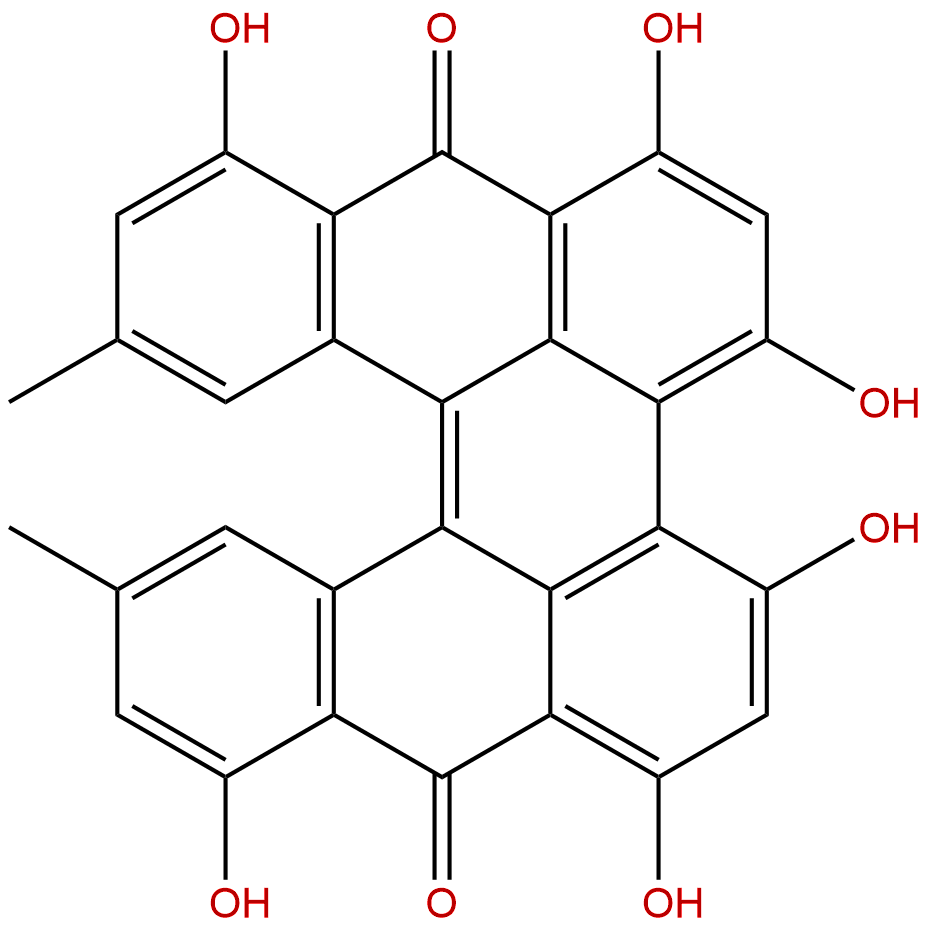
ProtohypericinCAS No.:548-03-8
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1808 |
| Formula: | C30H18O8 |
| Mol Weight: | 506.466 |
Product name: Protohypericin
Synonym name:
Catalogue No.: BP1808
Cas No.: 548-03-8
Formula: C30H18O8
Mol Weight: 506.466
Botanical Source:
Physical Description:
Type of Compound: Anthraquinones
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Protohypericin exhibits photocytotoxicity.
References:
Planta Med. 1999 Dec;65(8):719-22.
Photocytotoxicity of protohypericin after photoconversion to hypericin.
In the present study, Protohypericin was synthesised in order to compare its intrinsic photocytotoxicity with that of hypericin.
METHODS AND RESULTS:
The experimental work was performed in specific filtered light conditions that prevented both an unintended photoconversion of Protohypericin and photosensitization of the cells. Assessing the photocytotoxicity as a function of irradiation time, it was found that the photocytotoxicity of both compounds converged after a long irradiation time (i.e., 15 min), while the difference between the photocytotoxicities was maximal after a short irradiation time (i.e., 1 min).
CONCLUSIONS:
Since this could not be accounted for by a redistribution of Protohypericin during irradiation, and the different irradiation times corresponded to different degrees of photoconversion of Protohypericin into hypericin, the results clearly suggest that Protohypericin exhibits intrinsically a dramatically lower photoactivity as compared to hypericin.
J AOAC Int. 2005 Nov-Dec;88(6):1607-12.
Simultaneous determination of protopseudohypericin, pseudohypericin, protohypericin, and hypericin without light exposure.
St. John's wort products are commonly standardized to total naphthodianthrones and hyperforin. Determination of these marker compounds is complicated because of the photochemistry of the naphthodianthrones pseudohypericin and hypericin and the instability of hyperforin in solution.
METHODS AND RESULTS:
Protopseudohypericin and Protohypericin have been identified as naturally occurring naphthodianthrones and, when exposed to light, they are converted into pseudohypericin and hypericin, respectively. However, exposure to light and the resulting naphthodianthrone free-radical reactions oxidize hyperforin. A mathematical relationship between the response of the proto compound and the resulting naphthodianthrone can be established by comparing the analytical response of the proto compound in a solution protected from light with the increase in the analytical response of naphthodianthrone in the same solution after exposure to light. By mathematically converting the proto compounds to their respective products, exposure to light can be avoided while still including proto compounds in a single assay.
CONCLUSIONS:
The method presented here details the reporting of all significant naphthodianthrones, including protopseudohypericin and Protohypericin, without exposure to light. This approach includes the benefits of improved naphthodianthrone precision and protection of hyperforin from oxidation.
J Drug Target. 2015 Jun;23(5):417-26.
Radiopharmaceutical evaluation of (131)I-protohypericin as a necrosis avid compound.
Hypericin is a necrosis avid agent useful for nuclear imaging and tumor therapy. Protohypericin, with a similar structure to hypericin except poorer planarity, is the precursor of hypericin.
METHODS AND RESULTS:
In this study, we aimed to investigate the impact of this structural difference on self-assembly, and evaluate the necrosis affinity and metabolism in the rat model of reperfused hepatic infarction. Protohypericin appeared less aggregative in solution compared with hypericin by fluorescence analysis. Biodistribution data of (131)I-Protohypericin showed the percentage of injected dose per gram of tissues (%ID/g) increased with time and reached to the maximum of 7.03 at 24 h in necrotic liver by gamma counting. The maximum ratio of target/non-target tissues was 11.7-fold in necrotic liver at 72 h. Pharmacokinetic parameters revealed that the half-life of (131)I-Protohypericin was 14.9 h, enabling a long blood circulation and constant retention in necrotic regions. SPECT-CT, autoradiography, and histological staining showed high uptake of (131)I-Protohypericin in necrotic tissues.
CONCLUSIONS:
These results suggest that (131)I-Protohypericin is a promising necrosis avid compound with a weaker aggregation tendency compared with hypericin and it may have a broad application in imaging and oncotherapy.
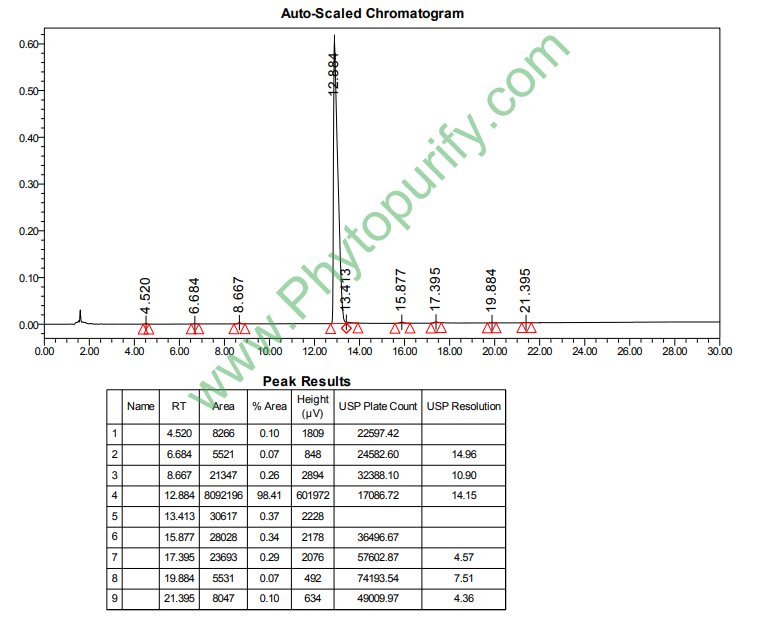
HPLC of Protohypericin