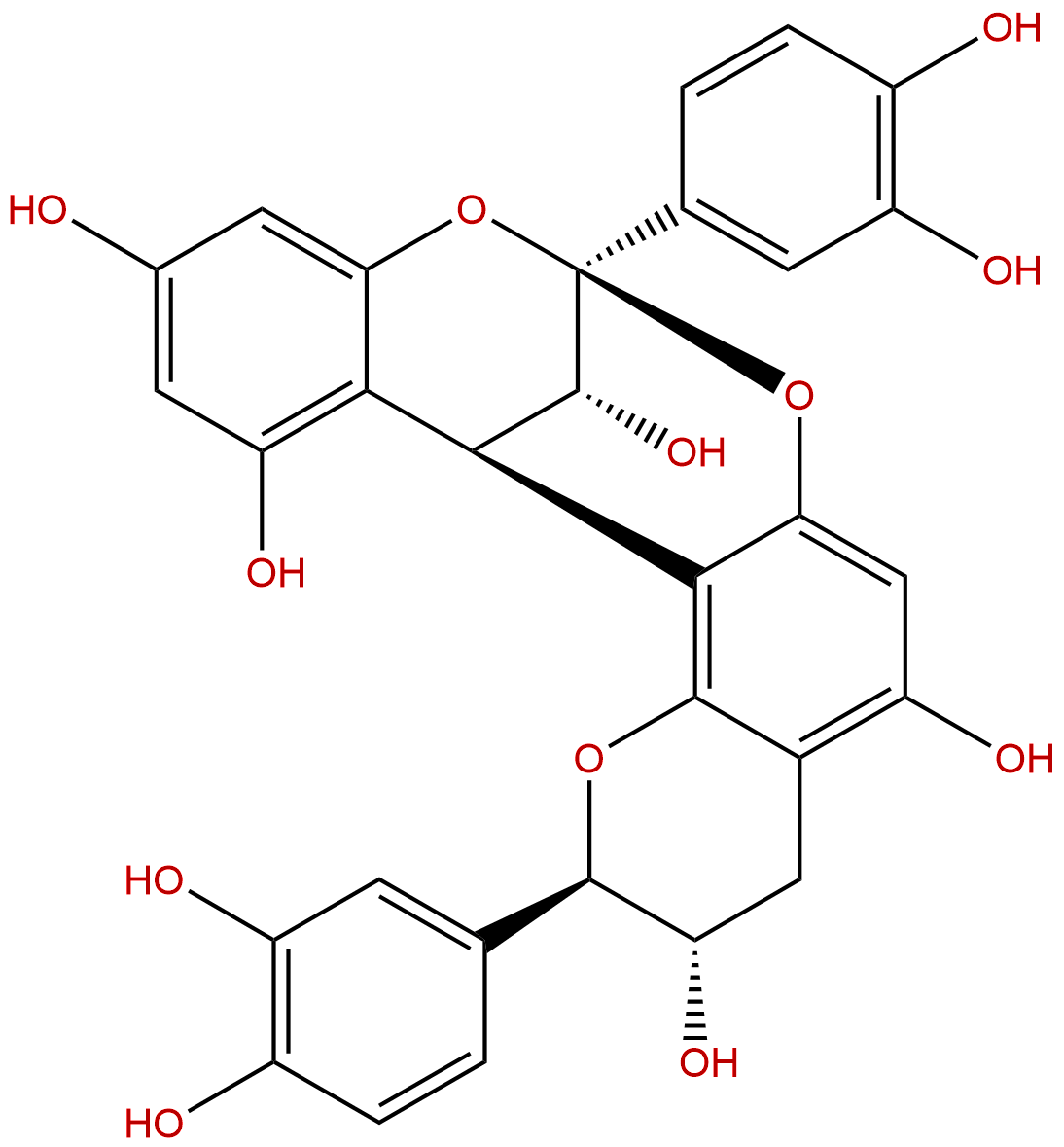
Procyanidin A1CAS No.:103883-03-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1832 |
| Formula: | C30H24O12 |
| Mol Weight: | 576.51 |
Product name: Procyanidin A1
Synonym name:
Catalogue No.: BP1832
Cas No.: 103883-03-0
Formula: C30H24O12
Mol Weight: 576.51
Botanical Source:
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Procyanidin A1 has antiallergic effects, it inhibits degranulation downstream of protein kinase C activation or Ca2⁺ influx from an internal store in RBL-2H3 cells. Procyanidin A1 shows a very high inhibition effect on LDL oxidation, with the IC50 value of 0.94 uM.
References:
Biosci Biotechnol Biochem. 2011;75(9):1644-8.
Effects of peanut-skin procyanidin A1 on degranulation of RBL-2H3 cells.
Peanut skin contains large amounts of polyphenols having antiallergic effects.
METHODS AND RESULTS:
We found that a peanut-skin extract (PSE) inhibits the degranulation induced by antigen stimulation of rat basophilic leukemia (RBL-2H3) cells. A low-molecular-weight fraction from PSE, PSEL, also had inhibitory activity against allergic degranulation. A main polyphenol in PSEL was purified by gel chromatography and fractionated by YMC-gel ODS-AQ 120S50 column. Electrospray ionization mass spectrometry (ESI-MS) analysis of the purified polyphenol gave m/z 599 [M+Na]⁺. Based on the results of ¹H-NMR, ¹³C-NMR spectra, and optical rotation analysis, the polyphenol was identified as Procyanidin A1. It inhibited the degranulation caused by antigen stimulation at the IC₅₀ of 20.3 µM. Phorbol-12-myristate-13-acetate (PMA) and 2,5,-di(tert-butyl)-1,4-hydroquinone (DTBHQ)-induced processes of degranulation were also inhibited by Procyanidin A1.
CONCLUSIONS:
These results indicate that peanut-skin Procyanidin A1 inhibits degranulation downstream of protein kinase C activation or Ca²⁺ influx from an internal store in RBL-2H3 cells.
Int J Mol Sci. 2017 Jun 26;18(7).
5-(3',4'-Dihydroxyphenyl-γ-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion.
Several metabolomics of polymeric flavan-3-ols have reported that proanthocyanidins are extensively metabolized by gut microbiota. 5-(3',4'-dihydroxyphenyl)-γ-valerolactone (DHPV) has been reported to be the major microbial metabolite of proanthocyanidins.
METHODS AND RESULTS:
We demonstrated that DHPV has stronger prevention effect on tumor necrosis factor (TNF)-α-stimulated adhesion of THP-1 human monocytic cells to human umbilical vein endothelial cells compared to its potential precursors such as Procyanidin A1, A2, B1 and B2, (+)catechin, (-)epicatechin and its microbial metabolites such as 3-(3,4-dihydroxyphenyl)propionic acid and 2-(3,4-dihydroxyphenyl)acetic acid. Mechanism study showed that DHPV prevents THP-1 monocyte-endothelial cell adhesion by downregulating TNF-α-stimulated expressions of the two biomarkers of atherosclerosis such as vascular cell adhesion molecule-1 and monocyte chemotactic protein-1, activation of nuclear factor kappa B transcription and phosphorylation of I kappa-B kinase and IκBα.
CONCLUSIONS:
We suggested that DHPV has higher potentiality in prevention of atherosclerosis among the proanthocyanidin metabolites.
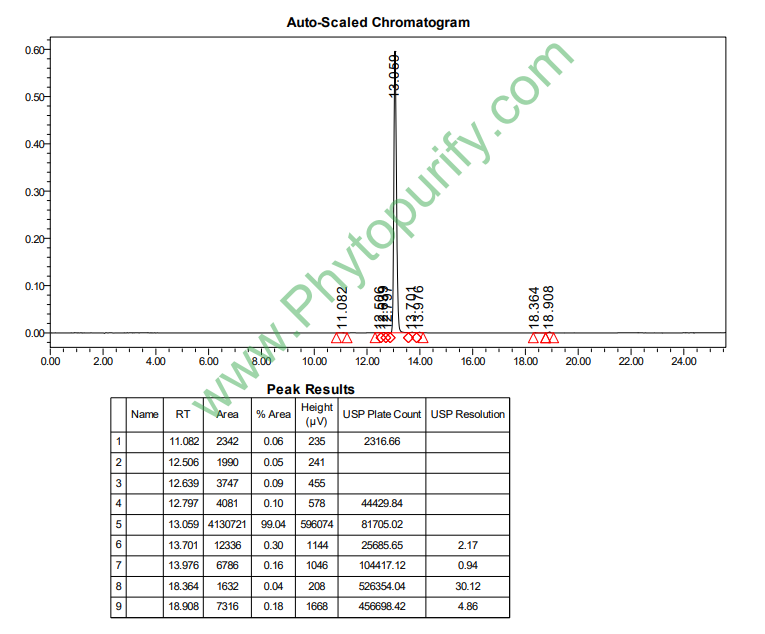
HPLC of Procyanidin A1