
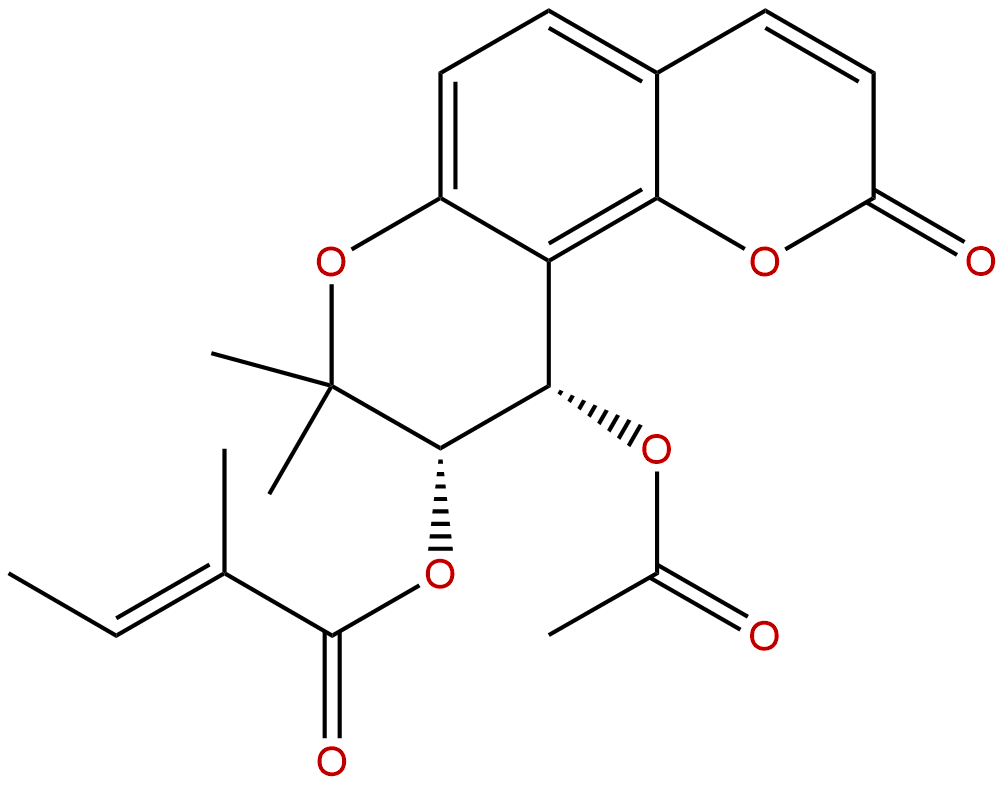
Praeruptorin ACAS No.:73069-27-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1133 |
| Formula: | C21H22O7 |
| Mol Weight: | 386.4 |
Synonym name:
Catalogue No.: BP1133
Cas No.: 73069-27-9
Formula: C21H22O7
Mol Weight: 386.4
Botanical Source: Peucedanum japonicum and Peucedanum praeruptorum
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
(+)-Praeruptorin A exerts distinct relaxant effects on isolated rat aorta rings, which may be mainly attributed to nitric oxide synthesis catalyzed by endothelial nitric oxide synthase.
References:
Chem Biol Interact. 2010 Jul 30;186(2):239-46.
(+/-)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis.
METHODS AND RESULTS:
(+)-Praeruptorin A showed more potent relaxation than (-)-praeruptorin A against KCl- and phenylephrine-induced contraction of rat isolated aortic rings with intact endothelium. Removal of the endothelium remarkably reduced the relaxant effect of (+)-Praeruptorin A but not that of (-)-praeruptorin A. Pretreatment of aortic rings with N(omega)-nitro-L-arginine methyl ester (L-NAME, an inhibitor of nitric oxide synthase) or methylene blue (MB, a soluble guanylyl cyclase inhibitor) resulted in similar changes of the relaxant effects of the two enantiomers to endothelium removal. Molecular docking studies also demonstrated that (+)-Praeruptorin A was in more agreement to nitric oxide synthase pharmacophores than (-)-praeruptorin A. On the other hand, the two enantiomers of praeruptorin A could slightly attenuate the contraction of rat aortic rings induced by internal Ca(2+) release from sarcoplasmic reticulum (SR).
CONCLUSIONS:
These findings indicated that (+)-Praeruptorin A and (-)-praeruptorin A exerted distinct relaxant effects on isolated rat aorta rings, which might be mainly attributed to nitric oxide synthesis catalyzed by endothelial nitric oxide synthase.