Picfeltarraenin IBCAS No.:97230-46-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3314 |
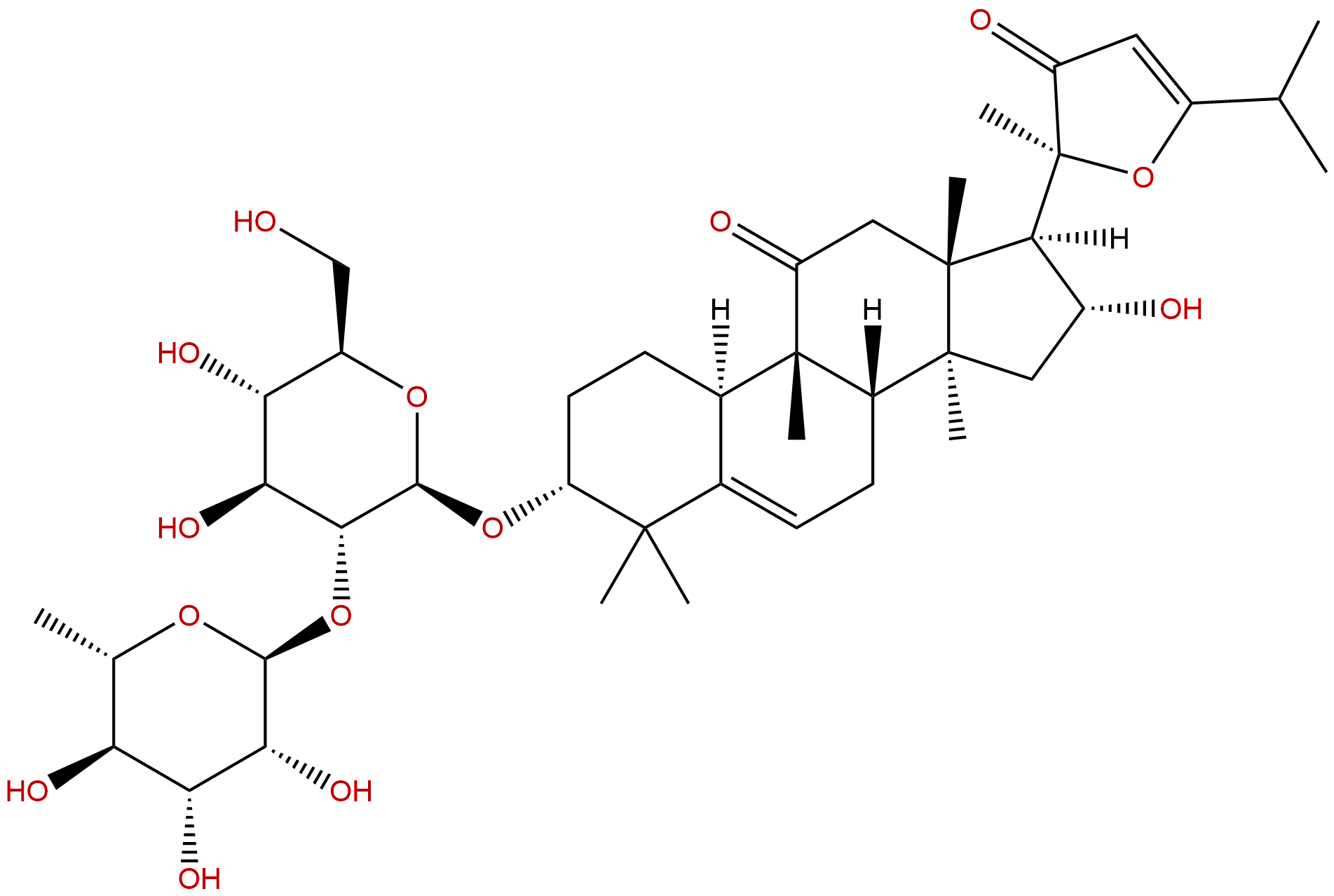
| Formula: | C42H64O14 |
| Mol Weight: | 792.96 |
Product name: Picfeltarraenin IB
Synonym name:
Catalogue No.: BP3314
Cas No.: 97230-46-1
Formula: C42H64O14
Mol Weight: 792.96
Botanical Source:
Physical Description: Powder
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Picfeltarraenin IA and Picfeltarraenin IB are Potential PI3K and EGFR Inhibitors, they also show stronger AChE inhibition than the known AChE inhibitor Tacrine.
References:
J Nat Prod. 1998 Jun 26;61(6):757-61.
Complement-inhibiting cucurbitacin glycosides from Picria fel-terrae.
Four cucurbitacin glycosides were isolated from Picriafel-terrae and identified by MS and NMR spectroscopy as picfeltarraenin IA (1), Picfeltarraenin IB (2), picfeltarraenin IV (4), and a new compound picfeltarraenin VI (3) (picfeltarraegenin I 3-O-beta-D-xylopyranoside).
METHODS AND RESULTS:
All four compounds acted as inhibitors on both the classical and alternative pathways of the complement system, with compound 3 exhibiting the highest inhibitory activity (IC50 29 +/- 2 microM and 21 +/- 1 microM, respectively). Compounds 1-4 showed no antiviral, antibacterial, or antifungal activities.
CONCLUSIONS:
Picfeltarraenin IA and Picfeltarraenin IB were tested in an in vitro human tumor cell line panel, but displayed no cytotoxic activity.
Pharmacogn Mag. 2013 Oct-Dec; 9(Suppl 1): S25–S31.
Bioassay- and liquid chromatography/mass spectrometry-guided acetylcholinesterase inhibitors from Picriafel-terrae
Picria fel-terrae is a traditional Chinese medicine.
METHODS AND RESULTS:
A new approach to the search for acetylcholinesterase (AChE) inhibitors from Picria fel-terrae is presented. Bioassay- and LC-MS-guided fractionation of the ethyl acetate extract was from traditional Chinese medicine P.fel-terrae. Following primary extraction, the ethyl acetate extracts fraction of P.fel-terrae showed strong AChE inhibitory activities. So the sample was separated using highperformance liquid chromatography (HPLC). The effluent was split towards two identical 96-well fraction collectors, and the presence of the biologically interesting portion and chromatographic fractions could be readily detected by analyzing selected ion chromatograms through an electrophoresis-electrospray ionization mass spectrometry (ESIMS) system for accurate mass measurement. One 96-well plate was used for a bioassay (AChE-inhibitory assay) and detected the bioactivity and position of the relevant peak in the chromatogram. The positive well in the second 96-well plate was used for identification by LC-(+) ESIMS.
CONCLUSIONS:
As abovementioned, the AChE inhibitory constituents from P.fel-terrae by LC-bioassay-ESIMS were rapid identified. Liquid chromatography/ mass spectrometry (LC-MS) screening detected the presence of six active compounds, identified as picfeltarraenin IA (1), Picfeltarraenin IB (2), picfeltarraenin IV (3), picfeltarraenin X (4), picfeltarraenin XI (5), and one unknown compound. The structures were further determined by 13C NMR. The six compounds expressed stronger AChE inhibition than the known AChE inhibitorTacrine. Above all, the value of this LC-bioassay-ESIMS methodology is highlighted by the finding and structure elucidation of the active constituents from many other structural families of natural products.
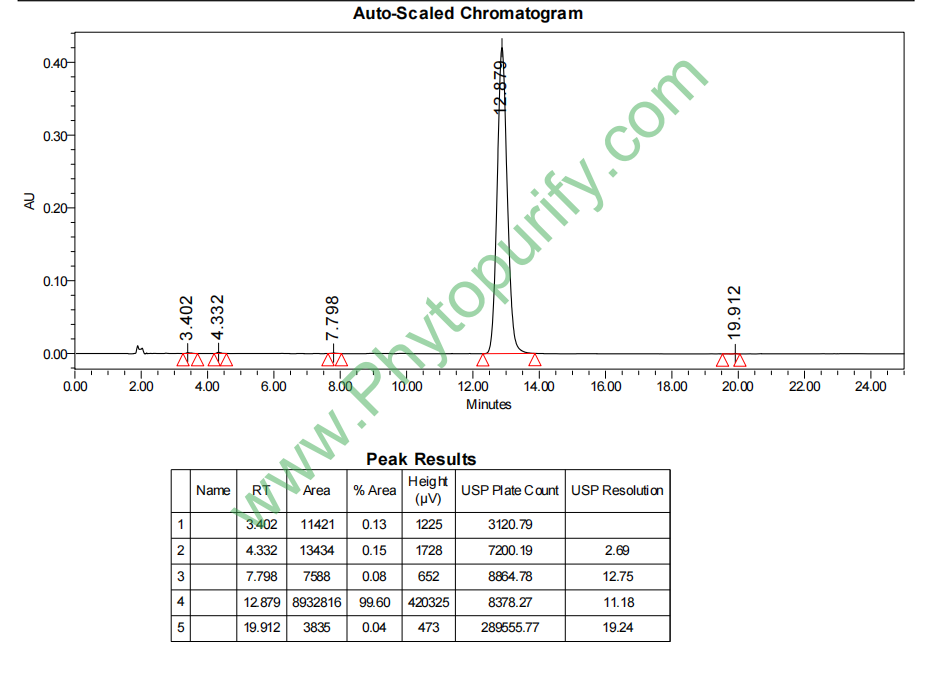
HPLC of Picfeltarraenin IB