
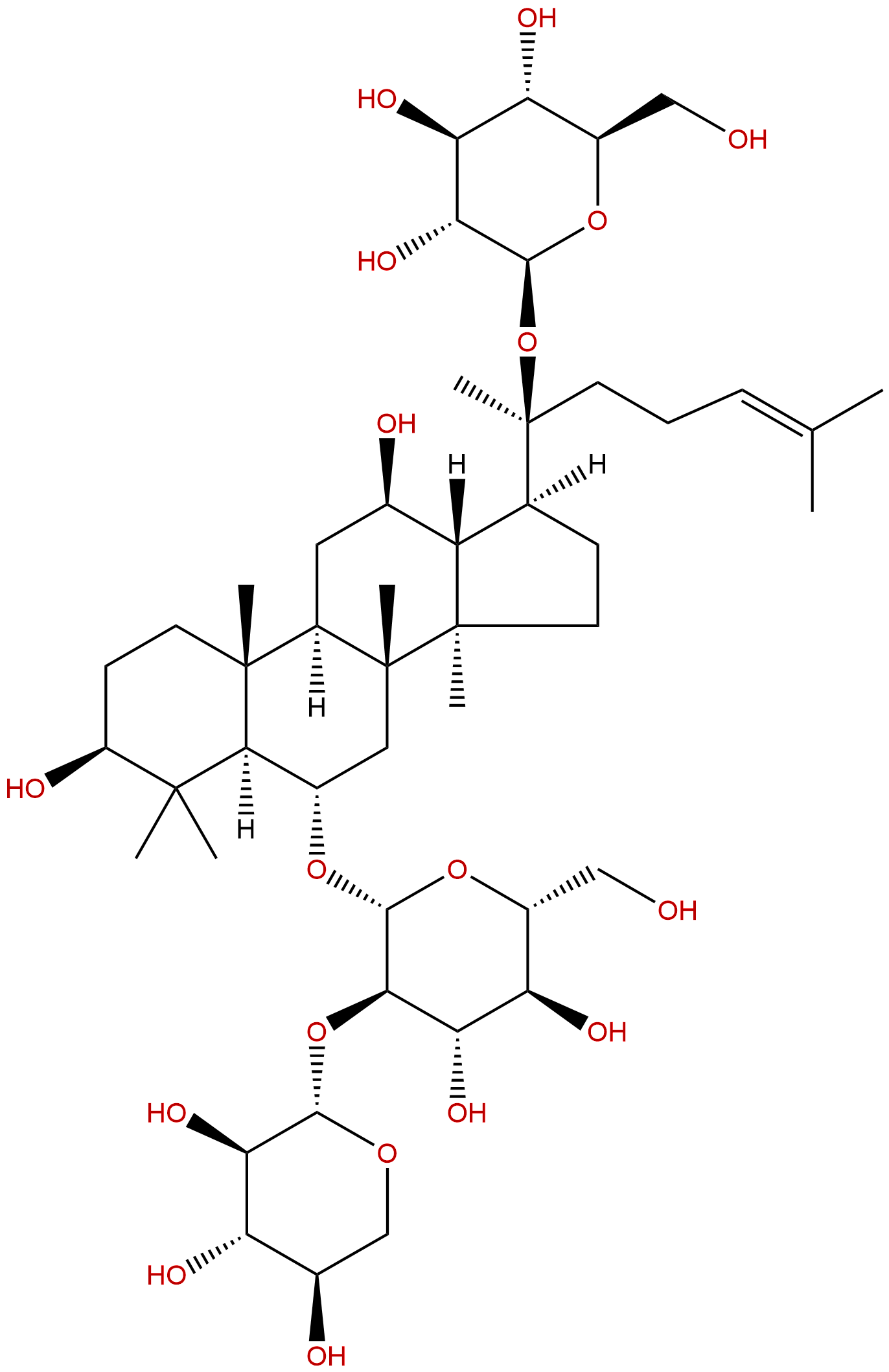
Notoginsenoside R1CAS No.:80418-24-2
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1010 |
| Formula: | C47H80O18 |
| Mol Weight: | 933.139 |
Product name: Notoginsenoside R1
Synonym name:
Catalogue No.: BP1010
Cas No.: 80418-24-2
Formula: C47H80O18
Mol Weight: 933.139
Botanical Source: Panax notoginseng(Burk.)F.H.Chen
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Notoginsenoside R1(NR1) is the main ingredient with cardiovascular activity in Panax notoginseng, which has some neuronal protective, antihypertensive,antioxidant, anti-inflammatory, antiapoptotic, and immune-stimulatory activities. NR1 can counteract endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo. NR1 inhibits TNF-α-induced PAI-1 overexpression via extracellular signal-related kinases (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling pathways, it attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation.
References:
Arterioscler Thromb Vasc Biol. 1997 Mar;17(3):465-74.
Notoginsenoside R1 counteracts endotoxin-induced activation of endothelial cells in vitro and endotoxin-induced lethality in mice in vivo.
In this study we investigated a possible counteracting activity Notoginsenoside R1 (NG-R1) on lipopolysaccharide (LPS)-induced effects in vitro and in vivo.
METHODS AND RESULTS:
The upregulation of plasminogen activator inhibitor-1 (PAI-1) antigen due to LPS (1 microgram/mL for 12 hours) in human umbilical vein endothelial cells (HUVECs) was prevented when the cells were incubated simultaneously with 100 micrograms/mL NG-R1 (PAI-1 antigen: LPS-treated cells, 969 +/- 54 ng/10(5) cells; control cells, 370 +/- 15 ng/10(5) cells; LPS + NG-R1-treated cells, 469 +/- 29 ng/10(5) cells; n = 6). The 2.5- and 3.4-fold (2.2- and 3.2-kb) increases in PAI-1 mRNA levels induced by LPS (1 microgram/mL for 6 hours) were reduced to 1.4- and 2.6-fold increases in the presence of both LPS and 100 micrograms/mL NG-R1. LPS-induced tissue factor (TF) activity in HUVECs was also counteracted when the cells were coincubated with both LPS and 100 micrograms/mL NG-R1 for 6 hours (TF activity: LPS-treated cells, 88.6 +/- 6.5 mU/10(6) cells; control cells, 0.7 +/- 0.01 mU/10(6) cells; LPS + NG-R1-treated cells, 56.0 +/- 1.9 mU/10(6) cells). The 26-fold increase in TF mRNA levels induced by LPS (1 microgram/mL for 2 hours) was reduced to a 13-fold increase in the presence of both LPS and 100 micrograms/mL NG-R1. PAI activity levels in the plasma of mice 4 hours after injection of LPS (10 ng/g body wt) increased 2.3-fold compared with a control group. In contrast, PAI activity from LPS + NG-R1 (1 microgram/g body wt NG-R1)-treated animals was at control level (PAI-1 activity: LPS-treated group, 11.3 +/- 3.1 U/mL; control group, 4.9 +/- 0.3 U/mL; LPS + NG-R1-treated group, 4.3 +/- 1.0 U/mL; n = 5 to 8). The production of TNF-alpha induced by 1 microgram/mL LPS by cultured human whole-blood cells was inhibited by 46% when the cells were incubated together with 100 micrograms/mL NG-R1. NG-R1 protected mice from the lethal effects of LPS. The 78% lethality induced by LPS/galactosamine was reduced to 23% when NG-R1 was administered simultaneously (P < .01 by chi 2 test). To extend this study to inflammatory cells, the effect of NG-R1 on LPS stimulation of the monocytic cell line THP-1 was investigated.
CONCLUSIONS:
NG-R1 inhibited the LPS-induced degradation of I kappa B-alpha and superinduced LPS-induced I kappa B-alpha mRNA, indicating that the effect of NG-R1 is not restricted to endothelial cells and is at least in part mediated by interference with the NF-kappa B/I kappa B-alpha pathway.
Int Immunopharmacol. 2014 Sep;22(1):151-9.
Notoginsenoside R1 attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation.
Progressive accumulation of amyloid-β (Aβ) is a pathological hallmark of Alzheimer's disease (AD). Aβ increases free radical production in neuronal cells, leading to oxidative stress and cell death.
METHODS AND RESULTS:
An intervention that would reduce Aβ-related neurotoxicity through free radical reduction could advance the treatment of AD. Notoginsenoside R1 (NR1), the major and most active ingredient in the herb Panax notoginseng, can reduce reactive oxygen species and confer some neuroprotective effects. Here, NR1 was applied in a cell-based model of Alzheimer's disease. Cell viability, cell death, reactive oxygen species generation, and mitochondrial membrane potential were assessed in cultured PC12 neuronal cells incubated with Aβ(25-35). In this model, Aβ was neurotoxic and induced necrosis and apoptosis; however, NR1 significantly counteracted the effects of Aβ by increasing cell viability, reducing oxidative damage (including apoptosis), restoring mitochondrial membrane potential, and suppressing stress-activated MAPK signaling pathways.
CONCLUSIONS:
These results promise a great potential agent for Alzheimer's disease and other Aβ pathology-related neuronal degenerative disease.
Free Radic Biol Med. 2006 May 1;40(9):1664-74.
Notoginsenoside R1 inhibits TNF-alpha-induced fibronectin production in smooth muscle cells via the ROS/ERK pathway.
The matrix fibronectin protein plays an important role in vascular remodeling. Notoginsenoside R1 is the main ingredient with cardiovascular activity in Panax notoginseng; however, its molecular mechanisms are poorly understood.
METHODS AND RESULTS:
We report that Notoginsenoside R1 significantly decreased TNF-alpha-induced activation of fibronectin mRNA, protein levels, and secretion in human arterial smooth muscle cells (HASMCs) in a dose-dependent manner. Notoginsenoside R1 scavenged hydrogen peroxide (H2O2) in a dose-dependent manner in the test tube. TNF-alpha significantly increased intracellular ROS generation and then ERK activation, which was blocked by Notoginsenoside R1 or DPI and apocynin, inhibitors of NADPH oxidase, or the antioxidant NAC. Our data demonstrated that TNF-alpha-induced upregulation of fibronectin mRNA and protein levels occurs via activation of ROS/ERK, which was prevented by treatment with Notoginsenoside R1, DPI, apocynin, NAC, or MAPK/ERK inhibitors PD098059 and U0126. Notoginsenoside R1 significantly inhibited H2O2-induced upregulation of fibronectin mRNA and protein levels and secretion; it also significantly inhibited TNF-alpha and H2O2-induced migration.
CONCLUSIONS:
These results suggest that Notoginsenoside R1 inhibits TNF-alpha-induced ERK activation and subsequent fibronectin overexpression and migration in HASMCs by suppressing NADPH oxidase-mediated ROS generation and directly scavenging ROS.
HPLC of Notoginsenoside R1

HNMR of Notoginsenoside R1
