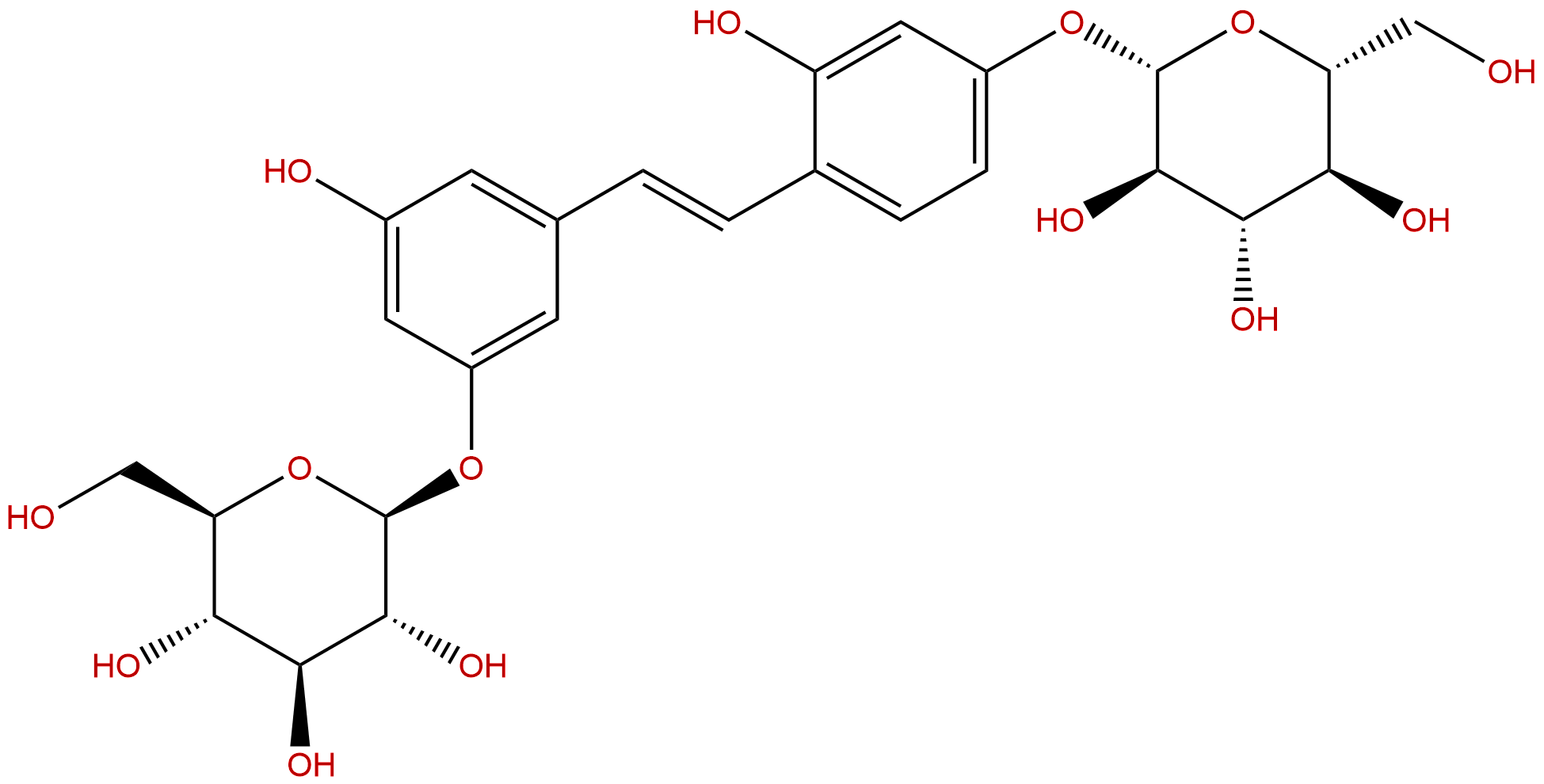
Mulberroside ACAS No.:102841-42-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0964 |
| Formula: | C26H32O14 |
| Mol Weight: | 568.528 |
Product name: Mulberroside A
Synonym name:
Catalogue No.: BP0964
Cas No.: 102841-42-9
Formula: C26H32O14
Mol Weight: 568.528
Botanical Source: Mori cotex
Physical Description:
Type of Compound: Stibene glucosides
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Mulberroside A, the major active anti-tyrosinase compound in the root bark extract of Morus alba L. (Moraceae), is widely employed as an active ingredient in whitening cosmetics. Mulberroside A has neuroprotective, analgesic, anti-inflammatory, antiapoptotic, uricosuric, nephroprotective, hypoglycemic, and antidiabetic effects.It also can protect mice against ethanol-induced hepatic damage.
References:
J Neurosci Res. 2014 Jul;92(7):944-54.
Mulberroside A protects against ischemic impairment in primary culture of rat cortical neurons after oxygen-glucose deprivation followed by reperfusion.
Mulberroside A is a natural polyhydroxylated stilbene compound present at relatively high abundance in the roots and twigs of Morus alba L. It is known for its nephroprotective, hypoglycemic, and antidiabetic effects. Because its metabolite, oxyresveratrol, possessed purported anti-inflammatory and neuroprotective effects, we proposed that Mulberroside A may elicit neuroprotective effects that can be used in the treatment of brain ischemic injury.
METHODS AND RESULTS:
Therefore, we decided to investigate the pharmacological properties of Mulberroside A in primary culture of rat cortical neurons after oxygen-glucose deprivation followed by reperfusion (OGD/R), evaluating its ability to counteract the hypoxia-ischemia impairment. The results showed that Mulberroside A elicited neuroprotective effects comparable to nimodipine. The mechanistic studies showed that Mulberroside A decreased the expressions of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 and inhibited the activation of NALP3, caspase-1, and nuclear factor-κB and the phosphorylation of extracellular signal-regulated protein kinases, the c-Jun N-terminal kinase, and p38, exhibiting anti-inflammatory antiapoptotic effects. Our results also further demonstrate that the proinflammatory cytokines of IL-1β, IL-6, and TNF-α are promising targets for treatment of cerebral ischemic injury.
CONCLUSIONS:
Although further investigation is required for its development, all of these findings led us to speculate that Mulberroside A is a candidate for the treatment of ischemic stroke, which would act as a multifactorial neuroprotectant.
Planta Med. 2011 May;77(8):786-94.
Mulberroside a possesses potent uricosuric and nephroprotective effects in hyperuricemic mice.
Mulberroside A is a major stilbene glycoside of MORUS ALBA L. (Moraceae), which is effectively used for the treatment of hyperuricemia and gout in traditional Chinese medicine. We examined whether Mulberroside A had effects on renal urate underexcretion and dysfunction in oxonate-induced hyperuricemic mice and investigated the potential uricosuric and nephroprotective mechanisms involved.
METHODS AND RESULTS:
Mulberroside A at 10, 20, and 40 mg/kg decreased serum uric acid levels and increased urinary urate excretion and fractional excretion of uric acid in hyperuricemic mice. Simultaneously, it reduced serum levels of creatinine and urea nitrogen (10-40 mg/kg), urinary N-acetyl- β-D-glucosaminidase activity (10-40 mg/kg), β₂-microglobulin (10-40 mg/kg) and albumin (20-40 mg/kg), and increased creatinine clearance (10-40 mg/kg) in hyperuricemic mice. Furthermore, Mulberroside A downregulated mRNA and protein levels of renal glucose transporter 9 (mGLUT9) and urate transporter 1 (mURAT1), and upregulated mRNA and protein levels of renal organic anion transporter 1 (mOAT1) and organic cation and carnitine transporters (mOCT1, mOCT2, mOCTN1, and mOCTN2) in hyperuricemic mice. This is the first study demonstrating that Mulberroside A exhibits uricosuric and nephroprotective effects mediated in part by cooperative attenuation of the expression alterations of renal organic ion transporters in hyperuricemic mice.
CONCLUSIONS:
These data suggest that Mulberroside A may be a new drug candidate for the treatment of hyperuricemia with renal dysfunction.
Environ Toxicol Pharmacol. 2008 Nov;26(3):325-30.
Protective function of cis-mulberroside A and oxyresveratrol from Ramulus mori against ethanol-induced hepatic damage.
The aim of the study was to investigate the protective effects of oxyresveratrol and cis-Mulberroside A isolated from Ramulus mori on the liver of mice intoxicated with ethanol.
METHODS AND RESULTS:
Animals were pretreated with different doses (30 and 60mg/kg of body weight) of oxyresveratrol and cis-Mulberroside A prior to the ethanol (9g/kg of body weight) orally for 7 days. Ethanol treatment induced the decrease of reduced glutathione level and antioxidant enzymes activities, the elevation of the lipid peroxidation and cytochrome P450 2E1 activity accompanied with the increase of iron concentration and mitochondrial permeability transition. Pretreatment with oxyresveratrol and cis-Mulberroside A restored the changes in the above parameters up to the basal level. The protective effects of the two active compounds were further supported by attenuation of the degree of tissue damage and the regulation of the expression of TNF-α.
CONCLUSIONS:
It could be concluded that oxyresveratrol and cis-Mulberroside A from R. mori could protect mice against ethanol-induced hepatic damage.
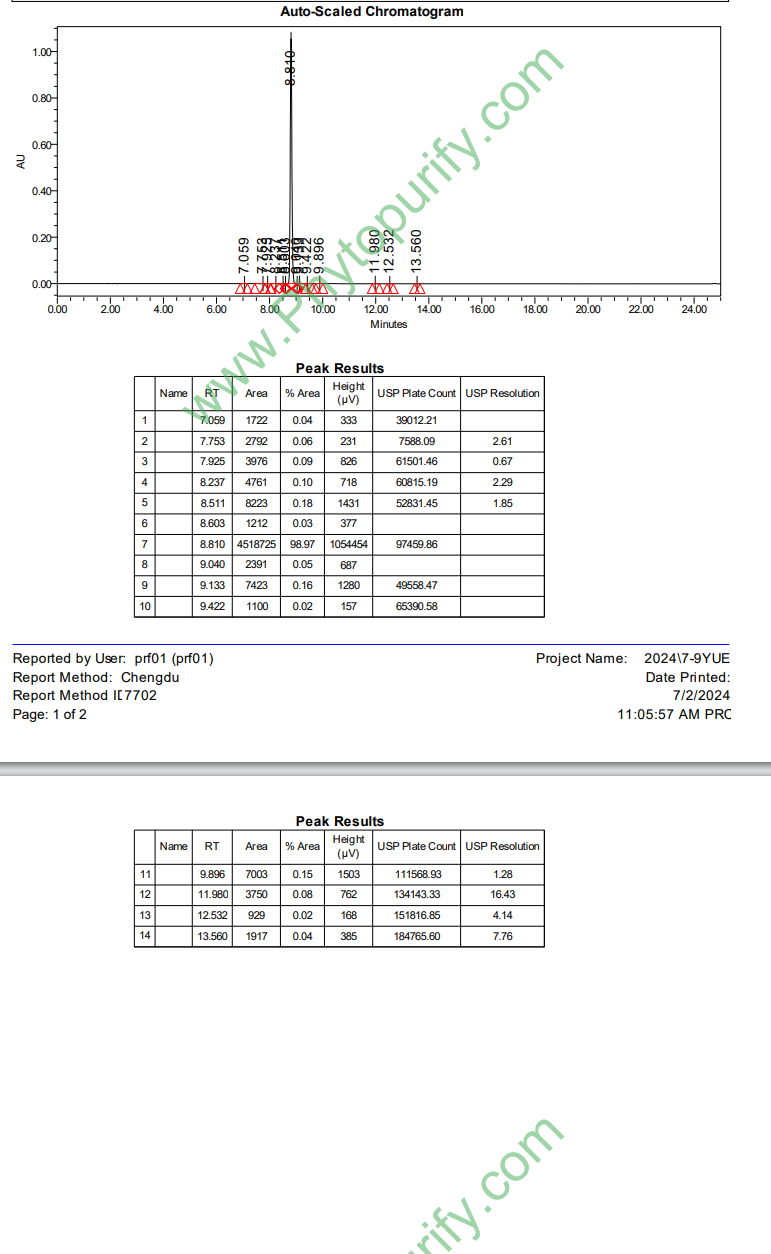
HPLC of Mulberroside A