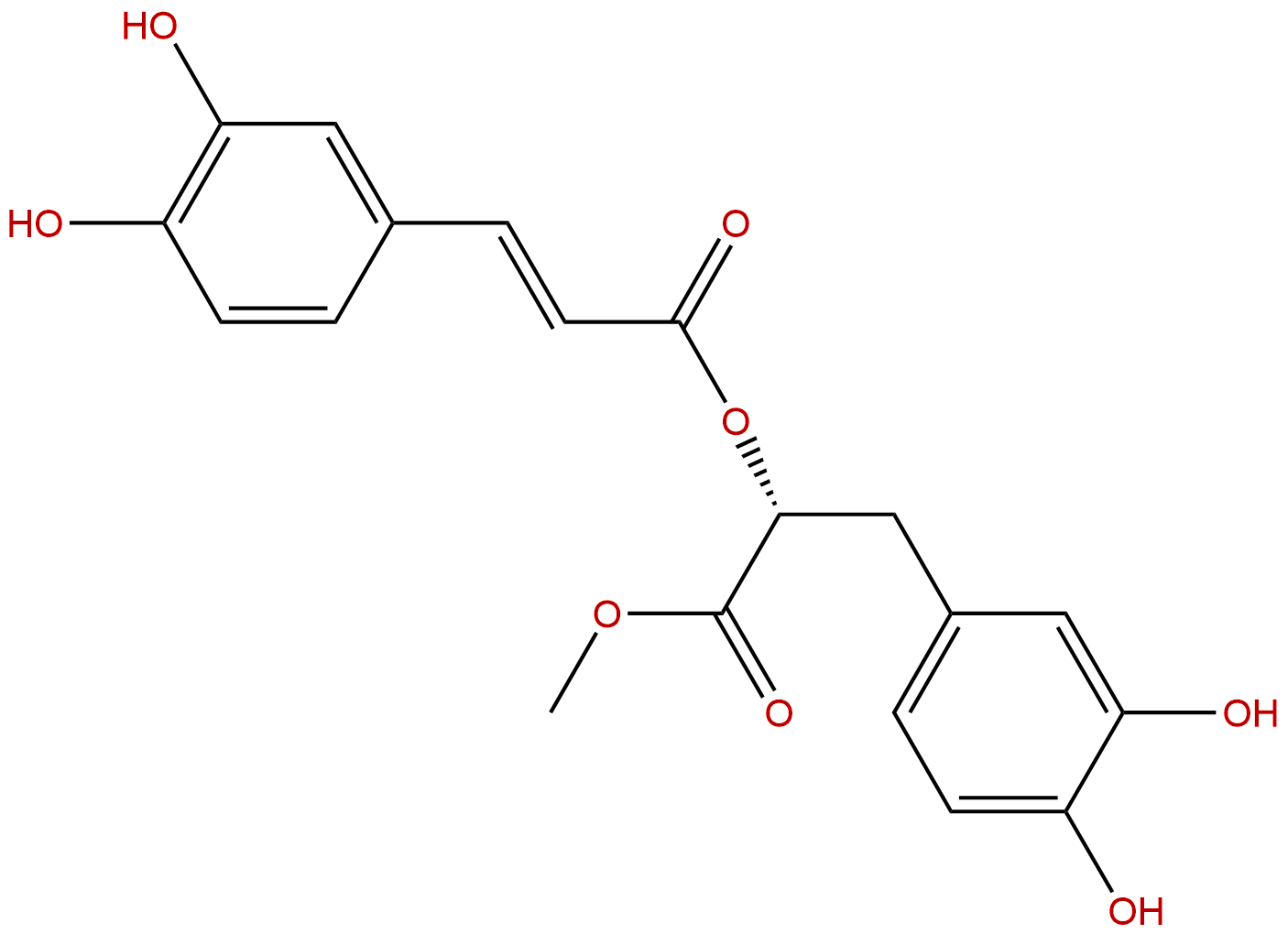
Methyl rosmarinateCAS No.:99353-00-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3256 |
| Formula: | C19H18O8 |
| Mol Weight: | 374.345 |
Product name: Methyl rosmarinate
Synonym name:
Catalogue No.: BP3256
Cas No.: 99353-00-1
Formula: C19H18O8
Mol Weight: 374.345
Botanical Source:
Physical Description: Powder
Type of Compound: Phenylpropanoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Methyl rosmarinate shows antioxidative, and antifungal activities. It has inhibitory activities against tyrosinase, α-glucosidase, and matrix metalloproteinase-1 (MMP-1).
References:
J Food Prot. 2013 Sep;76(9):1539-48.Impact of fatty acid chain length of rosmarinate esters on their antimicrobial activity against Staphylococcus carnosus LTH1502 and Escherichia coli K-12 LTH4263.
The effect of the addition of a newly synthesized series of rosmarinic acid (RA) estes (REs) and alcohols with chain lengths of 1, 4, 8, 10, 12, 16, and 18 carbons (RE1 to 18) on the growth behavior of Staphylococcus carnosus LTH1502 and Escherichia coli K-12 LTH4263 was investigated.
METHODS AND RESULTS:
An initial microtiter dilution assay indicated activity of compounds against S. carnosus LTH1502, whereas esters with chain lengths, RA, n-Methyl rosmarinate (RE1), n-dodecyl rosmarinate (RE12), and n-octadecyl rosmarinate (RE18) were used in a time-kill assay S. carnosus LTH1502. Compounds were added at 0.75 mM in the log phase, 5 mM in the exponential phase, 10 mM in the stationary phase. RA had no effect in the lag and exponential phase but decreased cell counts during the stationary phase. In contrast, RE1 and RE12 decreased cell number in all three phase, will RE12 reducing counts most rapidly. Addition of RE18 did not affect regardless of the growth phase.
CONCLUSIONS:
Appearance and physiological state of S. carnosus LTH1502 cells indicated difference in the way the compounds interacted with and damaged cells. Results were attributed to the different physicochemical properties of RA and its esters.Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2284-8.[Chemical constituents of Hyptis rhomboidea and their antifungal activity].
The present work is to investigate the chemical constitutions of Hyptis rhomboidea and their antifungal activities.
METHODS AND RESULTS:
The compounds were isolated by Toyopearl HW-40, Sephadex LH-20, MCI-Gel CHP-20, RP-18, PTLC and silica column chromatographic methods and subjected to evaluate some monomers antifungal activity of eight kinds of plant pathogenic bacteria. Eleven compounds were isolated and identified as ethyl caffeate (1), ursolic acid (2), oleanolic acid (3), vanillactic acid (4), Methyl rosmarinate (5), kaempferol 3-O-alpha-L-rhamnopyranosyl-(1 --> 6) -beta-D-glucopyranoside (6), kaempferol 3-O-alpha-L-rhamnopyranosyl-(1 --> 6)-beta-D-glucopyranoside (7), ilexgenin A (8), beta-amyrin (9), kaempferol 3-O-beta-D-glucopyranoside (astrgalin, 10) and cholest-5-ene-3beta, 4beta-diol (11). Compound 1 showed the strongest inhibitory effect on Sclerotinia sclerotiorum with the MIC 16.2 mg x L(-1), and compound 5 showed the strongest inhibitory effect on S. minor and Exserohilum turcicum with MIC 16.2, 8.1 mg x L(-1), respectively.
CONCLUSIONS:
All compounds were isolated from the H. rhomboidea for the first time, and compounds 1 and 5 showed antifungal activity.
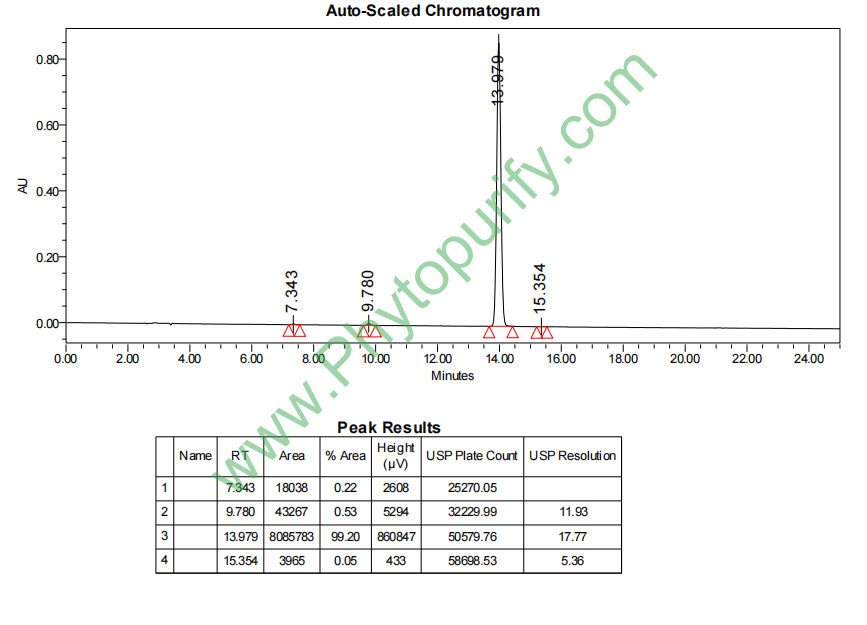
HPLC of Methyl rosmarinate