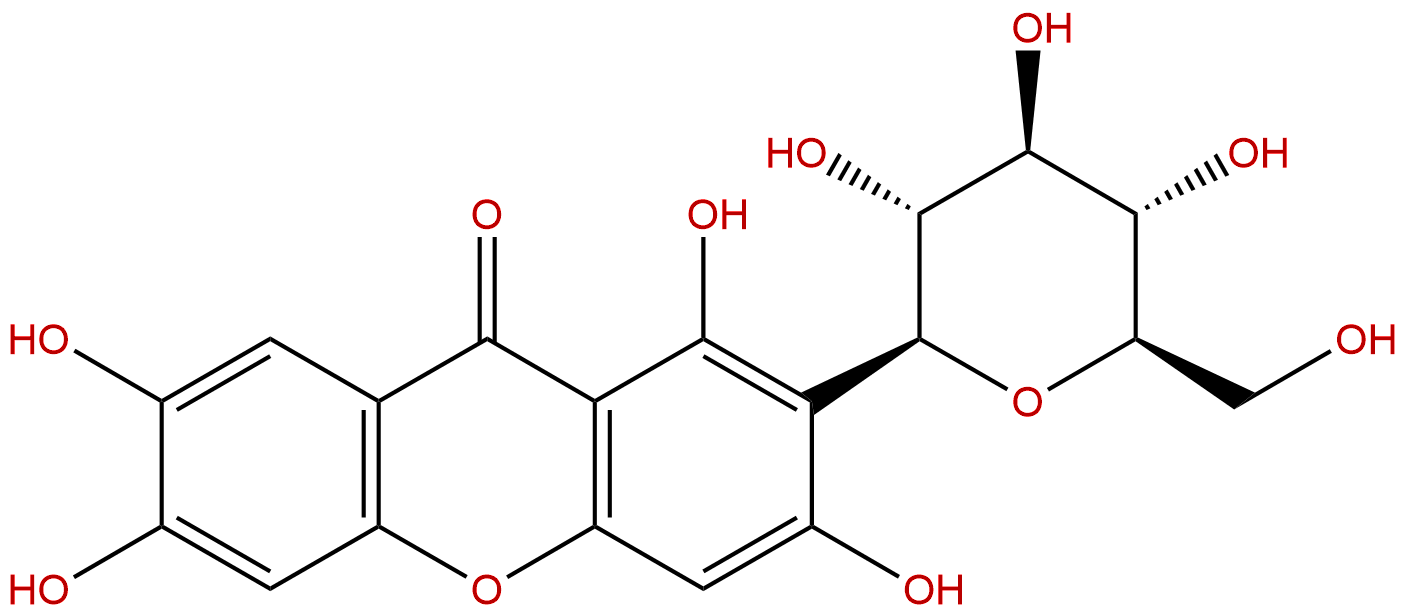
MangiferinCAS No.:4773-96-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0922 |
| Formula: | C19H18O11 |
| Mol Weight: | 422.342 |
Product name: Mangiferin
Synonym name: Euxanthogen; Chinomine; Alpizarin; Hedysaride; Mannipherin; Chedisaride; Shamimin
Catalogue No.: BP0922
Cas No.: 4773-96-0
Formula: C19H18O11
Mol Weight: 422.342
Botanical Source: Mangifera indica (mango) leaves, heartwood and stem bark and Iris spp., also from Salacia prinoides, Aphloia madagascariensis, Athyrium mesosorum, Anemarrhena asphodeloides, Belamcanda chinensis, Bombax spp., Hedysarum ussuriense and other pl
Physical Description: Powder
Type of Compound: Xanthones
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Mangiferin (MF) and its glucosides (mangiferin-7-O-beta-glucoside) (MG) isolated from Anemarrhena asphodeloides Bunge rhizome, have antidiabetic activity in KK-Ay mice,
MF and MG lower the blood glucose level of KK-Ay mice after oral administration, MF or MG improves hyperinsulinemia in KK-Ay mice; it seems likely that MF and MG exert their its antidiabetic activity by increasing insulin sensitivity.[1]
Mangiferin and pueraria glycoside (PG)-1 have an antioxidant activity, probably due to their ability to scavenge free radicals involved in initiation of lipid peroxidation; it can protect the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats; it can protect against 1-methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A cells.[2-4]
Mangiferin can reduce initial DNA damage and enhance DNA repair in the HPBLs exposed to 1 to 4 Gy γ-radiation and could serve as a protector against the radiation-induced DNA damage during planned and unplanned radiation exposures.[5]
Mangiferin has anti-inflammatory properties, can decrease inflammation and oxidative damage in rat brain after stress, it could be a new therapeutic strategy in neurological/neuropsychiatric pathologies in which hypothalamic/pituitary/adrenal (HPA) stress axis dysregulation, neuroinflammation, and oxidative damage take place in their pathophysiology.[6]
Mangiferin decreases plasma free fatty acids (FFA) levels through promoting FFA uptake and oxidation, inhibiting FFA and TG accumulations by regulating the key enzymes expression in liver through AMPK pathway, therefore, it is a possible beneficial natural compound for metabolic syndrome by improving FFA metabolism.[7]
Mangiferin has protective effects on iron-induced oxidative damage to rat serum and liver.[8]
Mangiferin attenuates osteoclastogenesis, bone resorption, and RANKL-induced activation of NF-κB and ERK.[9]
Mangiferin and vimang have anthelminthic and antiallergic activities.[10]
References:
[1] Ichiki H, Miura T, Kubo M, et al. Biol Pharm Bull. 1998 Dec;21(12):1389-90.
[2] Muruganandan S, Gupta S, Kataria M, et al. Toxicology, 2002, 176(3):165-73.
[3] Sato T , Kawamoto A , Tamura A ,et al. Chem Pharm Bull, 1992, 40(3):721-4.
[4] Amazzal L, Lap?tre A, Quignon F, et al. Neurosc Lett, 2007, 418(2):159-64.
[5] Jagetia G C, Venkatesha V A. Nutrition Res, 2006, 26(6):303-11.
[6] Márquez L, García-Bueno B, Madrigal J L M, et al. Eur J Nut, 2012, 51(6):729-39.
[7] Niu Y, Li S, Na L, et al. Plos One, 2012, 7(1):e30782.
[8] Pardo-Andreu G L, Barrios M F, Curti C, et al. Pharm Res, 2008, 57(1):79-86.
[9] Ang E, Liu Q, Qi M, et al. J Cell Biochem, 2011, 112(1):89-97.
[10] García D, Escalante M, Delgado R, et al. Phytother Res, 2003, 17(10):1203-8.
[11] Zhang X, Su B, Li J, et al. J Chromatogr Sci, 2012, 52(1):185-201.
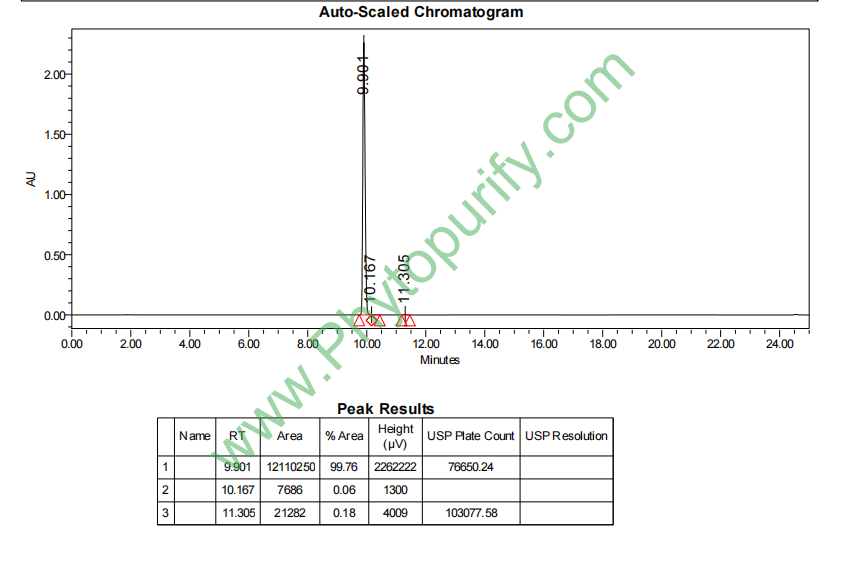
HPLC of Mangiferin