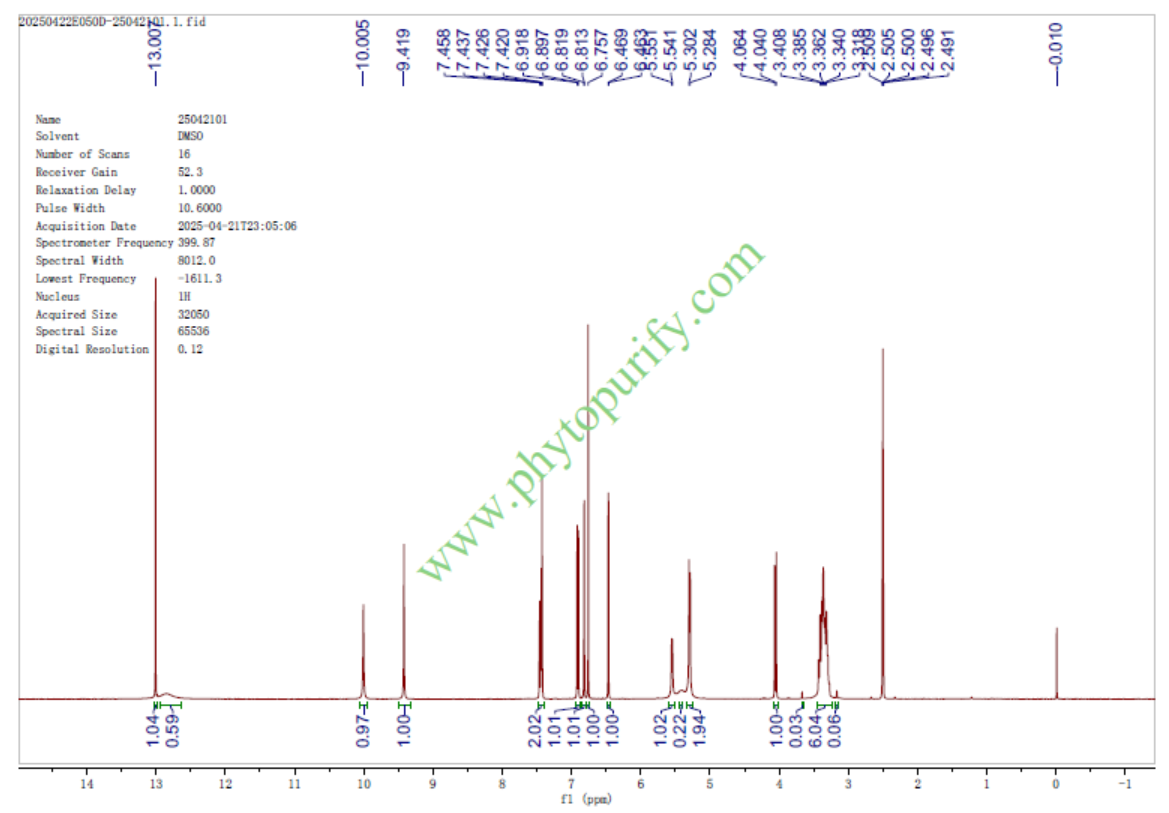
Luteolin 7-glucuronideCAS No.:29741-10-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1626 |
| Formula: | C21H18O12 |
| Mol Weight: | 462.363 |
Product name: Luteolin 7-glucuronide
Synonym name:
Catalogue No.: BP1626
Cas No.: 29741-10-4
Formula: C21H18O12
Mol Weight: 462.363
Botanical Source: " Isol. from Antirrhinum majus, Chrysanthemum sp., Scutellaria spp. and other plants"
Physical Description: Yellow powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Luteolin-7-O-glucuronide has anti-inflammatory activity. Luteolin 7-O-glucuronide shows potent α-glucosidase inhibitory effect with IC50 values of 14.7 uM, it also exhibits moderate α-amylase activity with IC50 values 61.5uM.Luteolin 7-O-glucuronide could inhibit Matrix Metalloproteinases (MMP) activities, with IC50s of 17.63, 7.99, 11.42, 12.85, 0.03 μM for MMP-1, MMP-3, MMP-8, MMP-9, MMP-13, respectively.
References:
J Med Food. 2015 Jan;18(1):83-94.
Quantification of major compounds from Ixeris dentata, Ixeris dentata Var. albiflora, and Ixeris sonchifolia and their comparative anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 cells.
The aim of the present study was to evaluate the comparative anti-inflammatory activities of Ixeris dentata (ID), Ixeris dentata var. albiflora (IDA), and Ixeris sonchifolia (IS) and to identify the main compounds present in extracts. The anti-inflammatory activity was evaluated through lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 murine macrophages. Five main compounds consisting of chlorogenic acid, caffeic acid, luteolin-7-O-glucoside, Luteolin-7-O-glucuronide, and luteolin were used for simultaneous high-performance liquid chromatography quantification.
METHODS AND RESULTS:
The total phenolic content present in ID (30 mg/g GAE), IDA (35.33 mg/g GAE), and IS (43.79 mg/g GAE) was correlated to the corresponding LPS-induced NO production inhibitory effect in RAW 264.7 cells as expressed with IC(50) values 26.19, 21.43, and 7.59 μg/mL, respectively. Luteolin-7-O-glucoside was found as the major compound in ID (8.76 mg/g dry weight) and IDA (10.35 mg/g dry weight) and Luteolin-7-O-glucuronide was the major compound in IS (34.66 mg/g dry weight). Luteolin 7-O-glucoside and Luteolin-7-O-glucuronide inhibited LPS-induced NO production with IC(50) values of 30 and 4.5 μM, respectively. Furthermore, luteolin, luteolin-7-O-glucoside, and luteolin- 7-O-glucuronide suppressed the expression of iNOS and COX-2, and t-BHP-induced ROS generation in LPS-stimulated RAW 264.7 cells.
CONCLUSIONS:
These results clearly showed that the anti-inflammatory potential of ID, IDA, and IS extract are primarily due to their contents of luteolin-7-O-glucoside and Luteolin-7-O-glucuronide, respectively.
Planta Med. 2017 Jul;83(11):901-911.
Correlating In Vitro Target-Oriented Screening and Docking: Inhibition of Matrix Metalloproteinases Activities by Flavonoids.
Metalloproteases are a family of zinc-containing endopeptidases involved in a variety of pathological disorders. The use of flavonoid derivatives as potential metalloprotease inhibitors has recently increased.Particular plants growing in Sicily are an excellent yielder of the flavonoids luteolin, apigenin, and their respective glycoside derivatives (7-O-rutinoside, 7-O-glucoside, and 7-O-glucuronide).
METHODS AND RESULTS:
The inhibitory activity of luteolin, apigenin, and their respective glycoside derivatives on the metalloproteases MMP-1, MMP-3, MMP-13, MMP-8, and MMP-9 was assessed and rationalized correlating in vitro target-oriented screening and in silico docking.The flavones apigenin, luteolin, and their respective glucosides have good ability to interact with metalloproteases and can also be lead compounds for further development. Glycones are more active on MMP-1, -3, -8, and -13 than MMP-9. Collagenases MMP-1, MMP-8, and MMP-13 are inhibited by compounds having rutinoside glycones. Apigenin and luteolin are inactive on MMP-1, -3, and -8, which can be interpreted as a better selectivity for both -9 and -13 peptidases. The more active compounds are apigenin-7-O-rutinoside on MMP-1 and luteolin-7-O-rutinoside on MMP-3. The lowest IC50 values were also found for apigenin-7-O-glucuronide, apigenin-7-O-rutinoside, and Luteolin-7-O-glucuronide. The glycoside moiety might allow for a better anchoring to the active site of MMP-1, -3, -8, -9, and -13.
CONCLUSIONS:
Overall, the in silico data are substantially in agreement with the in vitro ones (fluorimetric assay).
J Agric Food Chem. 2014 Jun 11;62(23):5290-5.
Bioavailability of hydroxycinnamic acids from Crepidiastrum denticulatum using simulated digestion and Caco-2 intestinal cells.
Hydroxycinnamic acids have antioxidant properties and potentially beneficial effects on human health. This study investigated the digestive stability, bioaccessibility, and permeability of hydroxycinnamic acids from Crepidiastrum denticulatum using simulated digestion and Caco-2 intestinal cells.
METHODS AND RESULTS:
The major compounds of C. denticulatum were determined to be four hydroxycinnamic acids [caftaric acid, chlorogenic acid, chicoric acid, and 3,5-di-O-caffeoylquinic acid (3,5-DCQA)] and one flavonoid (Luteolin-7-O-glucuronide) by high-performance liquid chromatography and electrospray ionization mass spectrometry. Hydroxycinnamic acids from C. denticulatum were rapidly released in the stomach and duodenum phase, maximizing the possibility of absorption in the intestinal Caco-2 cells. The digestive stability and bioaccessibility of hydroxycinnamic acids from C. denticulatum were markedly low after simulated digestion and remained minimal in the soluble fraction of the ileum phase. Unlike the four hydroxycinnamic acids, Luteolin-7-O-glucuronide was stable in terms of digestive stability and bioaccessibility during simulated digestion. The cell permeabilities (P(app A to B)/P(app B to A)) of caftaric acid (0.054) and chlorogenic acid (0.055) were higher than those of chicoric acid (0.011) and 3,5-DCQA (0.006) in general. That of Luteolin-7-O-glucuronide was not detectable, showing its low absorption in Caco-2 cells.
CONCLUSIONS:
These results indicate that the rapid release of hydroxycinnamic acids in the stomach and duodenum phase may increase the potential for absorption in Caco-2 cells, and that Luteolin-7-O-glucuronide, which was stable in terms of digestive stability and bioaccessibility, has relatively low absorption compared with hydroxycinnamic acids.
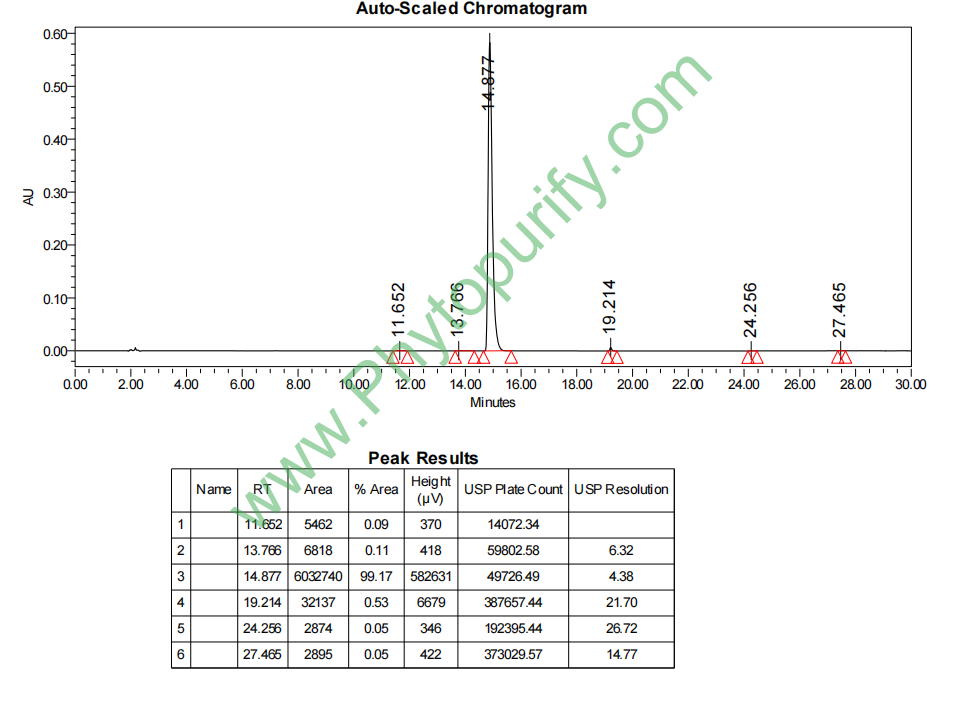
HPLC of Luteolin 7-glucuronide
HNMR of Luteolin 7-glucuronide