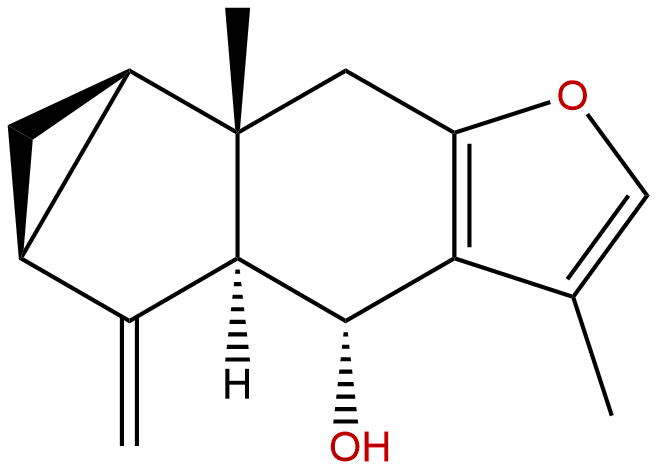
LindenenolCAS No.:26146-27-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3410 |
| Formula: | C15H18O2 |
| Mol Weight: | 230.307 |
Product name: Lindenenol
Synonym name:
Catalogue No.: BP3410
Cas No.: 26146-27-0
Formula: C15H18O2
Mol Weight: 230.307
Botanical Source:
Physical Description: Powder
Type of Compound: Sesquiterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Linderene, linderalactone and isolinderalactone inhibit the enzymes from both origins to the same extent.
References:
Biol Pharm Bull. 2002 Aug;25(8):1049-52.
Prolyl endopeptidase inhibitors from the roots of Lindera strychnifolia F. Vill.
We studied the PEP inhibitory constituents of the roots of Lindera strychnifolia F. VILL and isolated two known tannins, epicatechin (1) and aesculitannin B (2), and four known sesquiterpenes, Linderene (3), Linderene acetate (4), linderalactone (5) and isolinderalactone (6) as inhibitors.
METHODS AND RESULTS:
On the inhibitory activities of six compounds against PEP from Flavobacterium meningosepticum and that from rat brain supernatant, compounds 1, 2 and Linderene acetate inhibited the enzyme from Flavobacterium more strongly than that from rat brain supernatant. However, Linderene, linderalactone and isolinderalactone inhibited the enzymes from both origins to the same extent and furthermore, compound 6 was the strongest natural inhibitor against PEP from rat brain supernatant.
CONCLUSIONS:
The kinetic study of these inhibitors indicated that compounds 1, 2 are noncompetitive inhibitors and compounds Linderene,linderalactone,isolinderalactone are competitive inhibitors.
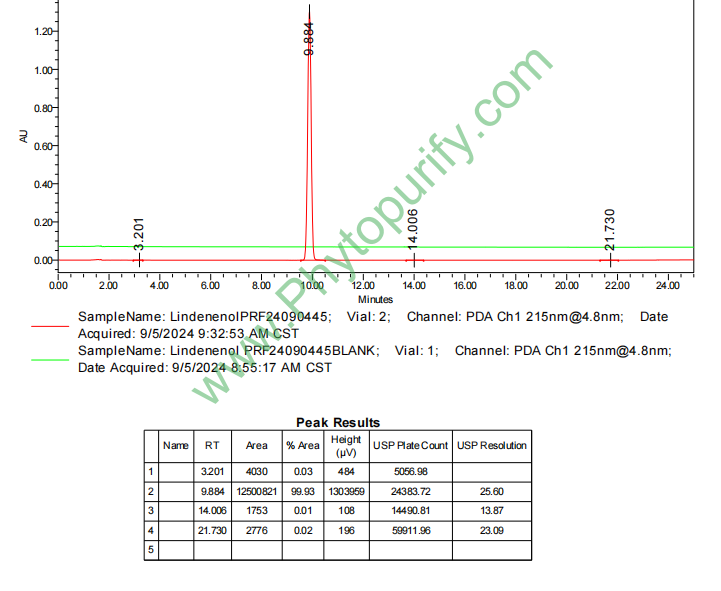
HPLC of Lindenenol