
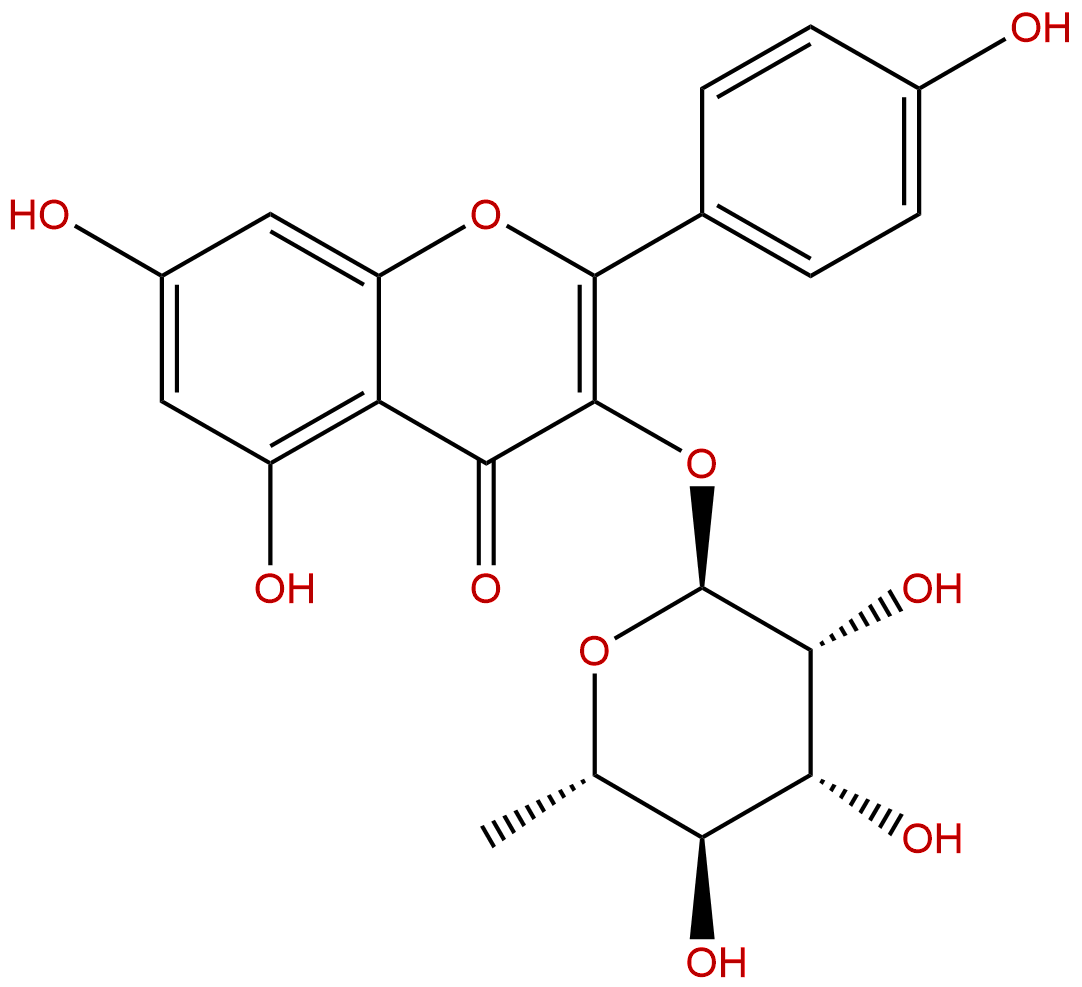
AfzelinCAS No.:482-39-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0818 |
| Formula: | C21H20O10 |
| Mol Weight: | 432.381 |
Product name: Afzelin
Synonym name: Kaempferol-3-rhamnoside; Kaempferin; Afzeloside
Catalogue No.: BP0818
Cas No.: 482-39-3
Formula: C21H20O10
Mol Weight: 432.381
Botanical Source: Afzelia sp. heartwood and many other plant spp. incl. ferns
Physical Description: Yellow powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Afzelin, isolated from Cornus macrophylla, has antibacterial effects on Pseudomonas aeruginosa, its minimum inhibitory concentration (MIC) is 31 μg/mL.[1]
Afzelin has several cellular activities such as DNA-protective, antioxidant, and anti-inflammatory as well as UV-absorbing activity and may protect human skin from UVB-induced damage by a combination of UV-absorbing and cellular activities.[2]
Afzelin has potenial anti-cancer activity against prostate cancer, the activity is due to inhibition of LIM domain kinase 021 expression, it can inhibit the proliferation of LNCaP and PC302cells, and block the cell cycle in the G002phase.[3]
Afzelin can attenuate asthma phenotypes is based on reduction of Th2 cytokine via inhibition of GATA-binding protein 3 transcription factor, which is the master regulator of Th2 cytokine differentiation and production.[4]
Afzelin promotes melanogenesis by occurs through increased MITF gene expression, which is mediated by activation of p38 MAPK, and suggest that afzelin may be useful as a protective agent against ultraviolet irradiation. [5]
References:
[1] Lee S Y, So Y J, Shin M S, et al. Molecules, 2014, 19(3):3173-80.
[2] Shin S W, Jung E, Kim S, et al. Plos One, 2013, 8(4):e61971-e61971.
[3] Zhu K C, Sun J M, Shen J G, et al. Oncol Lett, 2015, 10(4):2359-65.
[4] Zhou W, Nie X. Mol Med Rep, 2015, 12(1):71-6.
[5] Jung E, Jin H K, Mi O K, et al. Chem-Biol Interact, 2016, 254:167-72.
[6] Huo L, Chen X H, Cao Y, et al. Chinese J Pharm Anal, 2010(05):831-3.
HPLC of Afzelin
