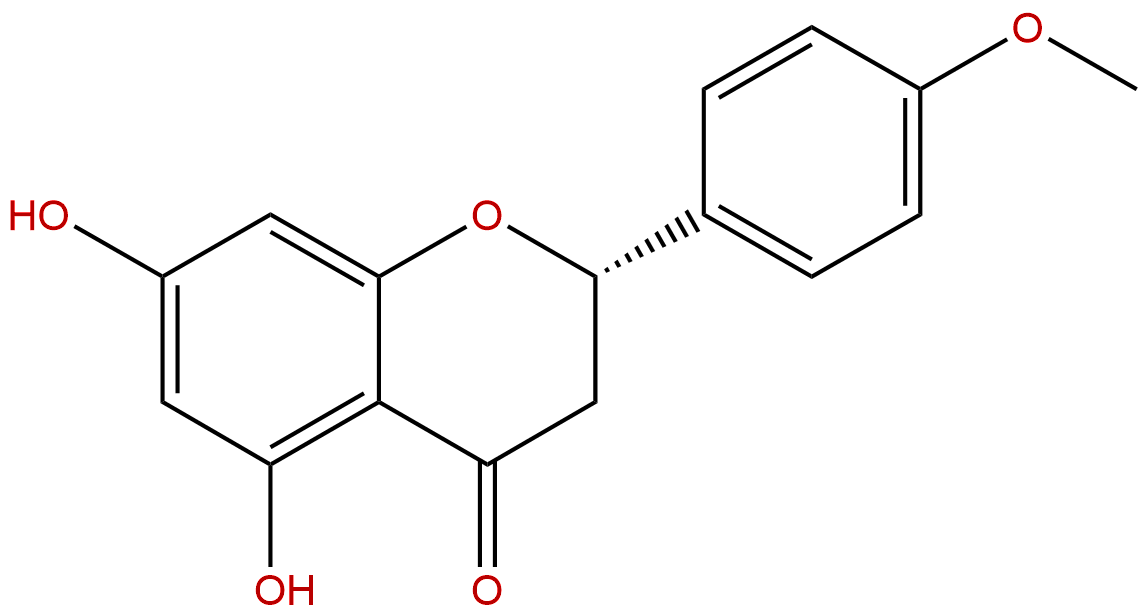
IsosakuranetinCAS No.:480-43-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3213 |
| Formula: | C16H14O5 |
| Mol Weight: | 286.283 |
Product name: Isosakuranetin
Synonym name:
Catalogue No.: BP3213
Cas No.: 480-43-3
Formula: C16H14O5
Mol Weight: 286.283
Botanical Source:
Physical Description: Powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Isosakuranetin is a plant exudate with known cytotoxic and fungicide properties, it may act on wheat root segments as an inhibitor of K+ permeation. Isosakuranetin is a TRPM3 blocker, significantly reduces the sensitivity of mice to noxious heat and PregS-induced chemical pain; it induced- inhibition of ERK1/2 and PI3K/AKT signaling pathways activate MITF and subsequent expression of Tyr, TRP1, and TRP2.
References:
Phytochemistry, 1997, 46(2):245-8.
The effect of isosakuranetin (5,7-dihydroxy 4′-methoxy flavanone) on potassium uptake in wheat root segments.
Isosakuranetin (ISK; 5,7-dihydroxy 4′-methoxy flavanone) is a plant exudate with known cytotoxic and fungicide properties.
METHODS AND RESULTS:
When tested on wheat root segments at a concentration of 70μM it inhibited K+ dependent H+ extrusion and net uptake of K+, while leaving the membrane potential (PD) unaltered. Fusicoccin (FC) + ISK treatment resulted in a slight membrane depolarization, while ISK alone did not alter O2 consumption or alternative oxidase activity. ISK did not increase the pyruvate content in incubated root tissues or inhibit Fe2+ uptake. The observed drop in K+ net uptake depended on a decrease in K+ influx into the cell, leading to the suggestion that ISK may act on wheat root segments as an inhibitor of K+ permeation.
CONCLUSIONS:
The lack of proton leakage and membrane disruption by ISK made this compound a weak candidate as a phytoalexin, and suggesting a major role as an allelopathic molecule.
Mol Pharmacol. 2013 Nov;84(5):736-50.
Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo.
Transient receptor potential melastatin 3 (TRPM3) is a calcium-permeable nonselective cation channel that is expressed in a subset of dorsal root (DRG) and trigeminal ganglia sensory neurons. TRPM3 can be activated by the neurosteroid pregnenolone sulfate (PregS) and heat. TRPM3⁻/⁻ mice display an impaired sensation of noxious heat and thermal hyperalgesia. We have previously shown that TRPM3 is blocked by the citrus fruit flavanones hesperetin, naringenin, and eriodictyol as well as by ononetin, a deoxybenzoin from Ononis spinosa.
METHODS AND RESULTS:
To further improve the tolerability, potency, and selectivity of TRPM3 blockers, we conducted a hit optimization procedure by rescreening a focused library that was composed of chemically related compounds. Within newly identified TRPM3 blockers, Isosakuranetin and liquiritigenin displayed favorable properties with respect to their inhibitory potency and a selective mode of action. Isosakuranetin, a flavanone whose glycoside is contained in blood oranges and grapefruits, displayed an IC₅₀ of 50 nM and is to our knowledge the most potent inhibitor of TRPM3 identified so far. Both compounds exhibited a marked specificity for TRPM3 compared with other sensory TRP channels, and blocked PregS-induced intracellular free Ca2⁺ concentration signals and ionic currents in freshly isolated DRG neurons. Furthermore, Isosakuranetin and previously identified hesperetin significantly reduced the sensitivity of mice to noxious heat and PregS-induced chemical pain.
CONCLUSIONS:
Because the physiologic functions of TRPM3 channels are still poorly defined, the development and validation of potent and selective blockers is expected to contribute to clarifying the role of TRPM3 in vivo.
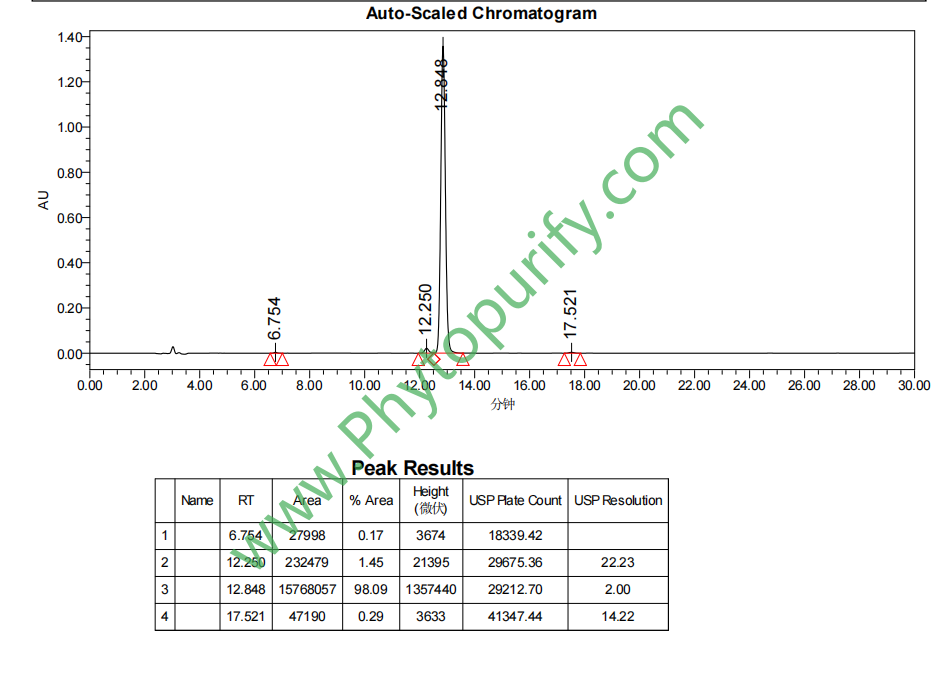
HPLC of Isosakuranetin