
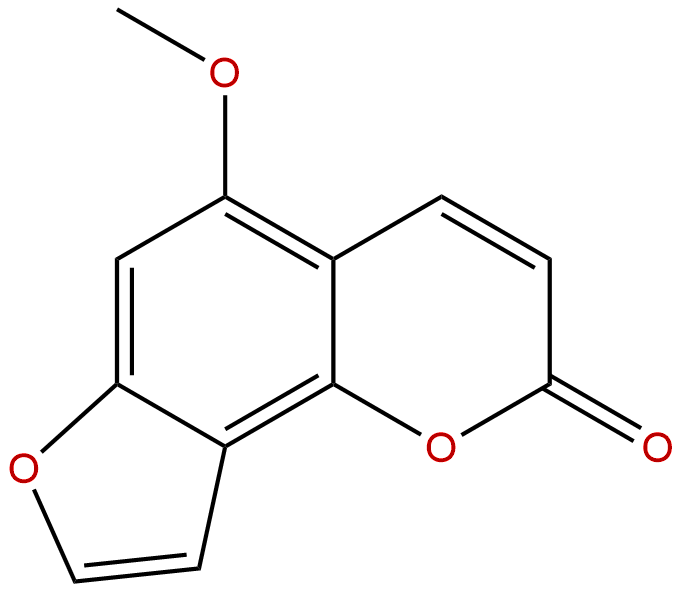
IsobergaptenCAS No.:482-48-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0775 |
| Formula: | C12H8O4 |
| Mol Weight: | 216.192 |
Synonym name: 5-Methoxyangelicin
Catalogue No.: BP0775
Cas No.: 482-48-4
Formula: C12H8O4
Mol Weight: 216.192
Botanical Source: roots of Heracleum spp. and Pimpinella saxifraga. Also from Pimpinella magna and Ruta pinnata
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Isobergapten is a plant growth regulating substance, it is the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum; it may form an important class of natural defensive agents against fungi.Isobergapten shows anti-proliferative activity and causes G2/M arrest at concentrations of 0.05-15.0 μM.
References:
J Ethnopharmacol. 2013 May 2;147(1):232-7.
The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.
Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity. To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum.
METHODS AND RESULTS:
A methanolic extract of Heracleum maximum roots was subjected to bioassay guided fractionation using the microplate resazurin assay (MRA) to assess inhibitory activity against Mycobacterium tuberculosis strain H37Ra. The antimycobacterial constituents were identified by NMR, MS and polarimetry. The polyacetylene (3R,8S)-falcarindiol and the furanocoumarins bergapten, Isobergapten, angelicin, sphondin, pimpinellin, isopimpinellin and 6-isopentenyloxyIsobergapten were isolated from the Heracleum maximum root extract. (3R,8S)-Falcarindiol and 6-isopentenyloxyIsobergapten exhibited MICs of 24 μM and 167 μM and IC50s of 6 μM and 27 μM against Mycobacterium tuberculosis H37Ra respectively. The remaining furanocoumarins bergapten, Isobergapten, angelicin, sphondin, pimpinellin, and isopimpinellin were less active, with MICs of 925, 1850, 2149, 1859, 812 and 1625 μM and IC50s of 125, 344, 350, 351, 389 and 406 μM.
CONCLUSIONS:
(3R,8S)-Falcarindiol, bergapten, Isobergapten, angelicin, sphondin, pimpinellin, isopimpinellin and 6-isopentenyloxyIsobergapten were identified as the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum. This work supports the ethnopharmacological use of Heracleum maximum by Canadian First Nations and Native American communities as a treatment for infectious diseases, specifically tuberculosis.
Phytochemistry, 1982, 21(9):2213-5.
Plant growth regulators from Heracleum lanatum.
The plant growth regulating substances, pimpinellin, isopimpinellin, bergapten, Isobergapten, vaginidiol, sphondin, scopoletin, umbelliferone, ferulic.
Ann. Appl. Biol., 1966, 57(3):501-8.
The fungitoxicities of plant furocoumarins.[Reference: WebLink]
SUMMARYA mixture of the furocoumarins pimpinellin, isopimpinellin, Isobergapten and sphondin isolated from Heracleum sphondylium root was toxic to Gloeosporium limetticola, Botryis cinerea, Sclerotinia fructigena and Stereum purpureum at 200 p.p.m. or less in nutrient medium. The extractive of the leaves of plants that contain furocoumarins suppressed in vivo growth of G. limetticola and B. cinerea at concentrations lower than the contents of the extractive in the leaves.
CONCLUSIONS:
Furocoumarins may form an important class of natural defensive agents against fungi; this possibility is discussed.