
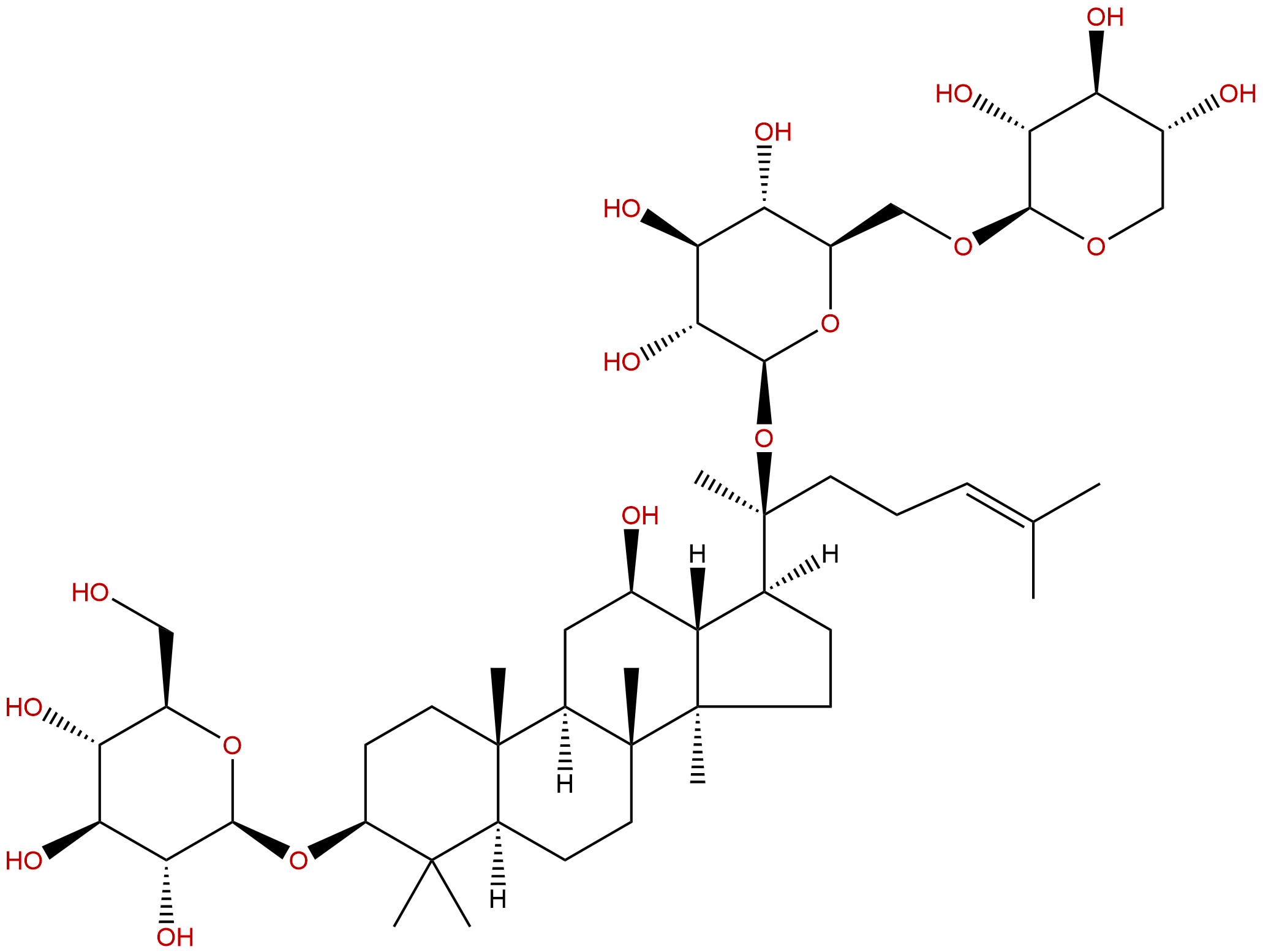
Gypenoside IXCAS No.:80321-63-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1790 |
| Formula: | C47H80O17 |
| Mol Weight: | 917.14 |
Product name: Gypenoside IX
Synonym name: Notoginsenoside Fd
Catalogue No.: BP1790
Cas No.: 80321-63-7
Formula: C47H80O17
Mol Weight: 917.14
Botanical Source: Panax notoginseng
Physical Description: White powder
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Gypenosides (Gyp, Stevenleaf) are triterpenoid saponins contained in an extract from Gynostemma pentaphyllum Makino, they induce apoptosis in human hepatoma cells through the up-regulation of Bax and Bak, and down-regulation of Bcl-2, release of mitochondrial cytochrome c and activation of caspase cascade.[1]
Gypenosides induce ER stress and production of reactive oxygen species and Ca 2+ , change the ratio of Bcl-2 and Bax, followed by the dysfunction of mitochondria, cause cytochrome c release, activation of caspase-3 before leading to apoptosis, these results provide information towards an understanding of the mechanisms by which Gyp induces cell cycle arrest and apoptosis in human tongue cancer cells.[2]
Gypenosides can inhibit invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis.[3]
Gypenosides can protect cortical cells by multiple antioxidative actions via enhancing intracellular GSH, suppressing glutamate-induced cytosolic Ca 2+ elevation and blocking glutamate-induced apoptosis, GPs imply their remarkable preventative and therapeutic potential in treatment of neurological diseases involving glutamate and oxidative stress.[4]
Gypenosides protect biomembranes from oxidative injury by reversing the decreased membrane fluidity of liver microsomes and mitochondria, increasing mitochondrial enzyme activity in vascular endothelial cells and decreasing intracellular lactate dehydrogenase leakage from these cells; the extensive antioxidant effect of GPs may be valuable to the prevention and treatment of various diseases such as atherosclerosis, liver disease and inflammation.[5]
References:
[1] Wang Q F, Chen J C, Hsieh S J, et al. Cancer Lett, 2002, 183(2):169-78.
[2] Chen J C, Lu K W, Tsai M L, et al. Oral Oncol, 2008, 45(3):273-83.
[3] Lu K W, Tsai M L, Chen J C, et al. Anticancer Res, 2008, 28(2A):1093-9.
[4] Shang L, Liu J, Zhu Q, et al. Brain Res, 2006, 1102(1):163-74.
[5] Li L, Jiao L, Lau B H. Cancer Biother, 1993, 8(3):263-72.
[6] Liu C, Zhang Q, Xie E, et al. Drug Standards of China, 2013, 14(1):7-9.
HPLC of Gypenoside IX
