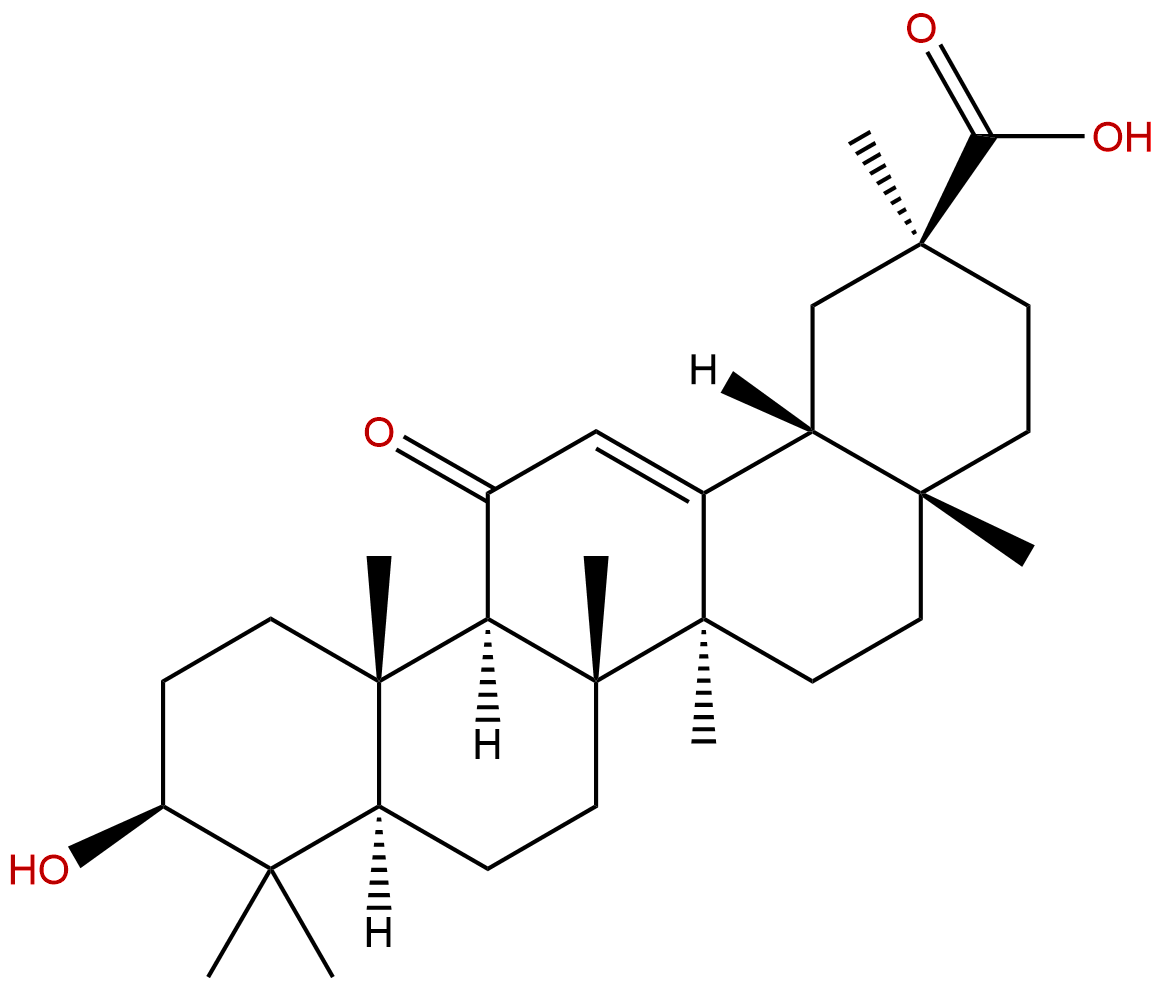
18β-Glycyrrhetinic acidCAS No.:471-53-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0681 |
| Formula: | C30H46O4 |
| Mol Weight: | 470.694 |
Product name: Glycyrrhetinic acid
Synonym name: Rhetinic acid; Uralenic acid
Catalogue No.: BP0681
Cas No.: 471-53-4
Formula: C30H46O4
Mol Weight: 470.694
Botanical Source: hexacoral Echinopora lamellosa. Aglycone from licorice (Glycyrrhiza glabra) and some other plants
Physical Description: Powder
Type of Compound: Triterpenoids
Purity: 95%~99%
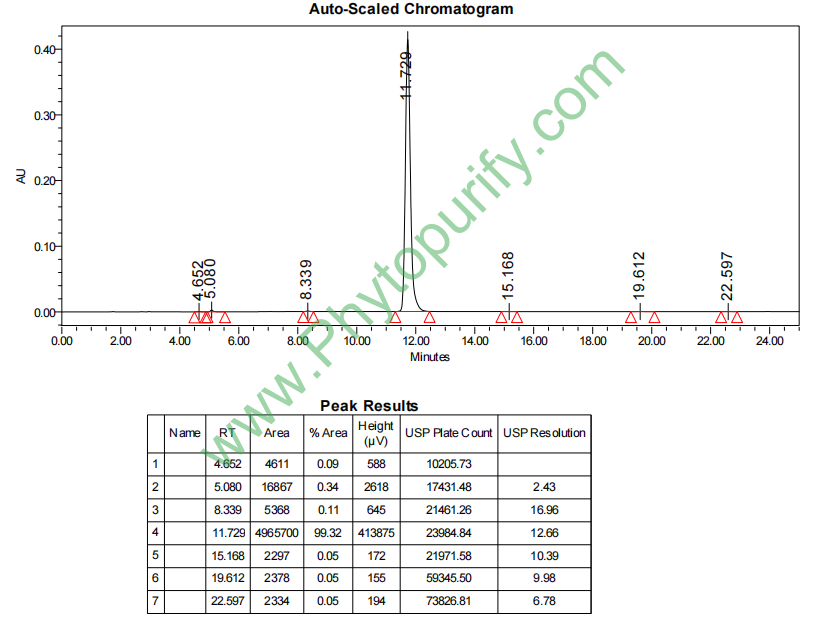
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Glycyrrhetinic acid, a hydrolysis product of one of the main constituents of licorice, the triterpene glycoside of glycyrrhizic acid, when added to rat liver mitochondria at micromolar concentrations induces swelling, loss of membrane potential, pyridine nucleotide oxidation, and release of cytochrome c and apoptosis inducing factor; indicates that glycyrrhetinic acid is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway.[1]
Glycyrrhetinic acid and some of its derivatives have anti-inflammatory activities.[2]
Glycyrrhizic acid shows no significant affinity for mineralocorticoid or glucocorticoid kidney receptor sites, has a low but definite affinity for mineralocorticoid receptors and thus appears to have a direct mineralocorticoid action. [3]
Glycyrrhetinic acid inhibits renal 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) and by that mechanism increases access of cortisol to the mineralocorticoid receptor that causes renal sodium retention and potassium loss, decreases plasma potassium concentrations in patients with anuria, and confers Na(+)-retaining properties on glucocorticoids and amplifies those of aldosterone and deoxycorticosterone. [4,5]
Glycyrrhetinic acid has antileukaemic activity.[6]
Glycyrrhetinic acid (10 microM) induces actin disruption and has tumor cell-selective toxic properties, and that its selectivity is superior to those of all the clinically available antitumor agents tested, suggests that it could be utilized as a promising chemopreventive and therapeutic antitumor agent. [7]
References:
[1] Salvi M, Fiore C D, Toninello A. Biochem Pharmacol, 2003, 66(66):2375-9.
[2] Finney R S H, ? G F S. J Pharm Pharmacol, 2011, 10(1):613-20.
[3] Ulmann A, Menard J, Corvol P. Endocrinology, 1975, 97(1):46-51.
[4] Morris D J, Semafuko W E, Latif S A, et al. Hypertension, 1992, 20(3):356-60.
[5] Serra A, Uehlinger D E, Ferrari P, et al. J Am Soc Nephrol, 2002, 13(1):191-6.
[6] Logemann W, Lauria F, Cudkowicz G, et al. Nature, 1960, 187(187):607-8.
[7] Yamaguchi H, Noshita T, Yu T. Eur J Med Chem, 2010, 45(7):2943-8.
[8] Ren P, Sun G, Ren P, et al. Asian J Trad Med, 2008(3):110-6.
HPLC of Glycyrrhetinic acid