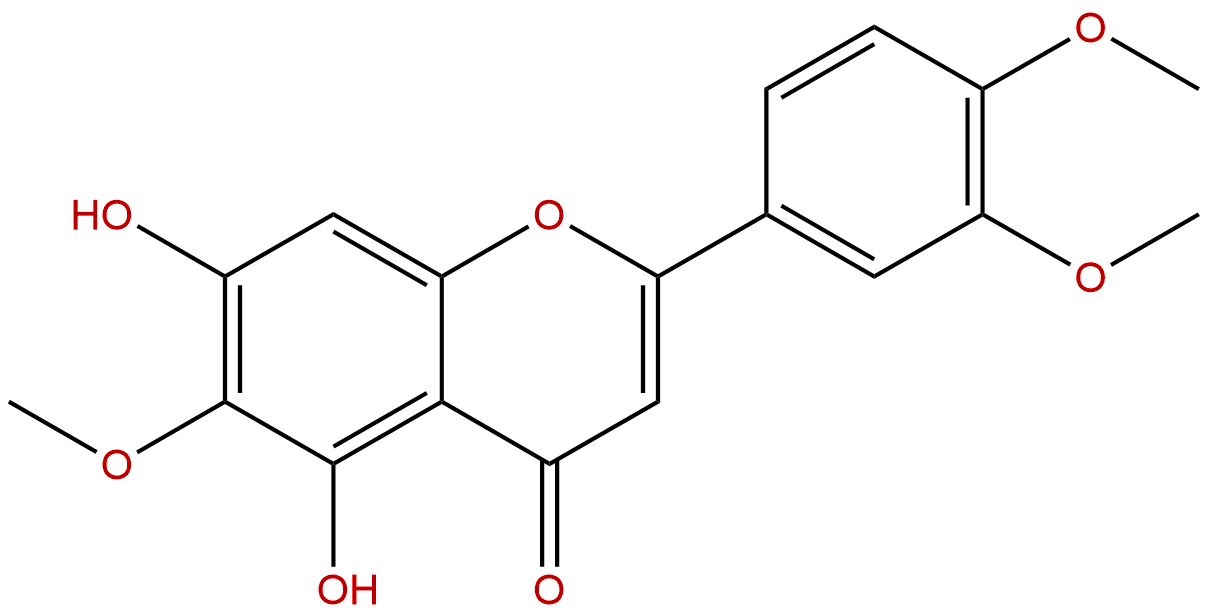
EupatilinCAS No.:22368-21-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0576 |
| Formula: | C18H16O7 |
| Mol Weight: | 344.319 |
Product name: Eupatilin
Synonym name:
Catalogue No.: BP0576
Cas No.: 22368-21-4
Formula: C18H16O7
Mol Weight: 344.319
Botanical Source: Eupatorium semiserratum, Tanacetum vulgare (tansy) and Liatris punctata
Physical Description: Yellow powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Eupatilinthe has anti-proliferative effect in MCF10A- ras cells, is associated with its blockade of cell cycle progression which appears to be attributable in part to inhibition of ERK1/2 activation.[1]
Eupatilin attenuates bile acid-induced hepatocyte apoptosis by suppressing bile acid-induced kinase activation, it might be therapeutically efficacious in a variety of human liver diseases associated with cholestasis.[2]
Eupatilin and jaceosidin have effects on cytochrome p450 enzyme activities in human liver microsomes, have potential pharmacokinetic drug interactions in vivo due to inhibition of CYP1A2 and CYP2C9.[3]
Eupatilin is a potent anti-atherogenic agent that inhibits PDGF-BB-induced proliferation and migration in HASMCs as well as aortic sprouting, which is likely mediated through the attenuation of PI3K, MKK3/6, and MKK4 activation.[4]
Eupatilin suppresses oxidative damage and reciprocally enhances extracellular matrix production in articular chondrocytes, making eupatilin a promising therapeutic option for the treatment.[5]
Eupatilin improves the acute hepatic IRI by reducing inflammation and apoptosis, it is a promising therapeutic agent against acute IR-induced hepatic damage.[6]
Eupatilin protects against tumor necrosis factor-α-mediated inflammation inhuman umbilical vein endothelial cells.[7]
Eupatilin induces Sestrin2-dependent autophagy to prevent oxidative stress.[8]
References:
[1] Kim D H, Na H K, Oh T Y, et al. Biochem Pharmacol, 2004, 68(6):1081-7.
[2] Su C P, Yoon J H, Kim W, et al. J Gastroenterol, 2006, 41(8):772-8.
[3] Ji H Y, Kim S Y, Kim D K, et al. Molecules, 2010, 15(9):6466-75.
[4] Son J E, Lee E, Seo S G, et al. Planta Med, 2013, 79(12):1009-16.
[5] Jeong J H, Moon S J, Jhun J Y, et al. Plos One, 2015, 10(6):e0130882.
[6] Lee H M, Jang H J, Kim S S, et al. Transpl P, 2016, 48(4):1226-33.
[7] Yu K, Li X M, Xu X L, et al. Int J Clin Exp Med, 2016, 8(12):22191-7.
[8] Jegal K H, Ko H L, Sang M P, et al. Apoptosis, 2016, 21(5):1-15.
[9] Zhou Q, Sun L L, Jiang B, et al. China Pharm, 2013, 24(47):4464-6.
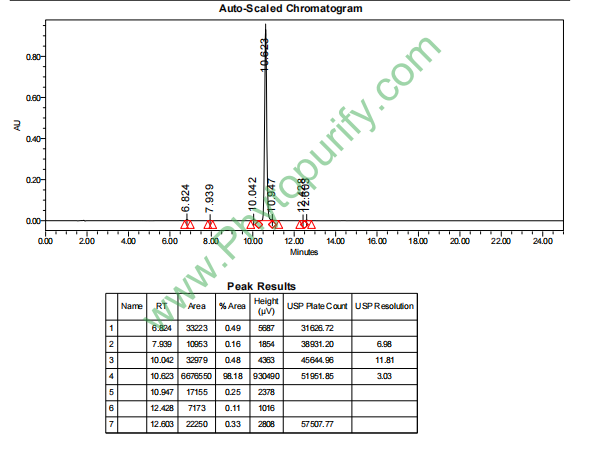
HPLC of Eupatilin