
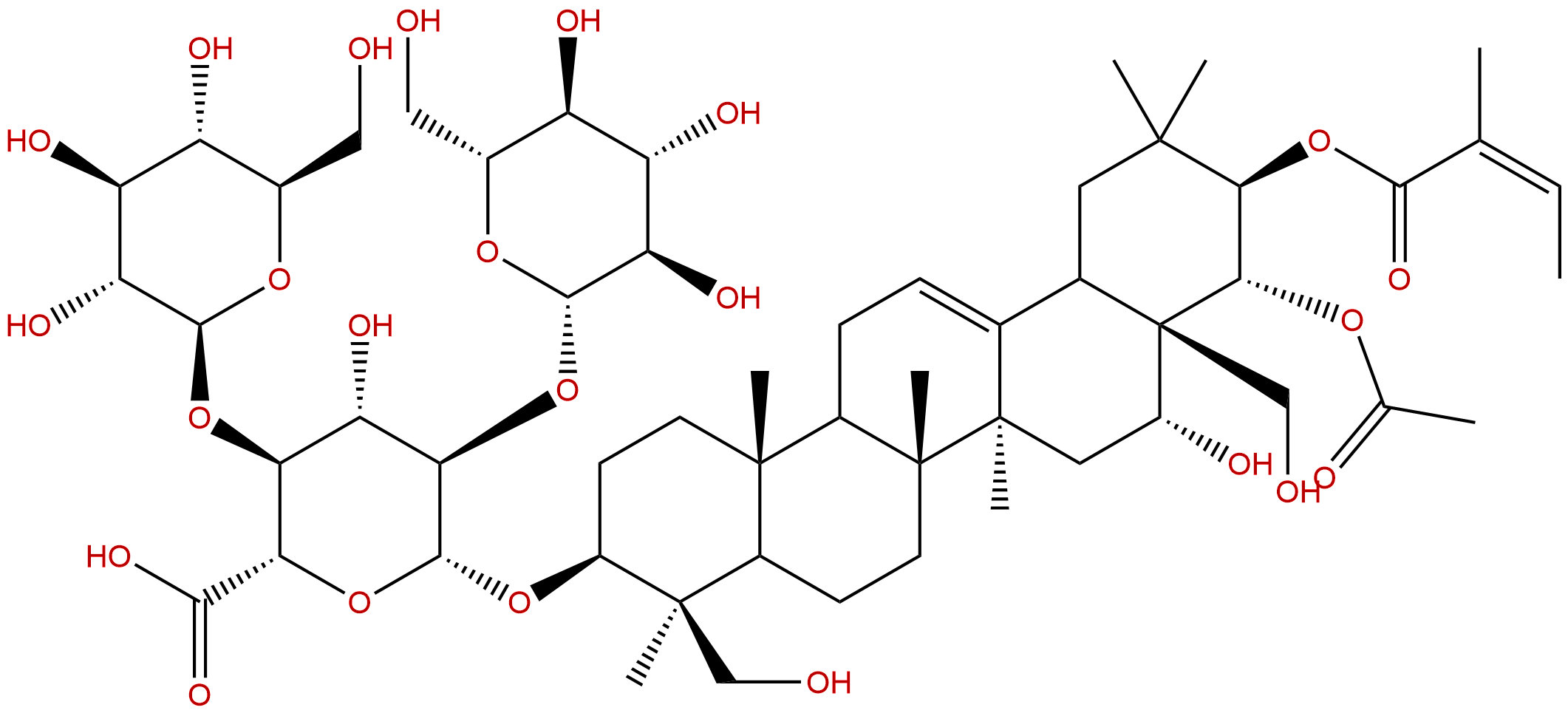
Escin IBCAS No.:26339-90-2
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0557 |
| Formula: | C55H86O24 |
| Mol Weight: | 1131.27 |
Product name: Escin IB
Synonym name: Aescin IB
Catalogue No.: BP0557
Cas No.: 26339-90-2
Formula: C55H86O24
Mol Weight: 1131.27
Botanical Source: Aesculi semen
Physical Description:
Type of Compound: Terpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Escin Ib inhibits gastric emptying, at least in part, mediated by capsaicin-sensitive sensory nerves, to stimulate the synthesis and/or release of dopamine, to act through central dopamine2 receptor, which in turn causes the release of PGs.
References:
Life Sci. 2000;66(23):2233-8.
Possible involvement of 5-HT and 5-HT2 receptors in acceleration of gastrointestinal transit by escin Ib in mice.
METHODS AND RESULTS:
We have reported previously that Escin IB accelerated gastrointestinal transit (GIT) in mice, and that its effect may be mediated by the release of endogenous prostaglandins (PGs) and nitric oxide (NO). In this study, the possible involvement of 5-HT and 5-HT receptors in the GIT acceleration of Escin IB was investigated in mice. The acceleration of GIT by Escin IB (25 or 50 mg/kg, p.o.) was attenuated by pretreatment with ritanserin (0.5-5 mg/kg, s.c., a 5-HT(2A/2C/2B) receptor antagonist), but not with MDL 72222 (1 and 5 mg/kg, s.c.) and metoclopramide (10 mg/kg, s.c.) (5-HT3 receptor antagonists) or tropisetron (1 and 10 mg/kg, s.c., a 5-HT(3/4) receptor antagonist). Furthermore, pretreatment with ketanserin (0.05-5 mg/kg, s.c.), haloperidol (1-5 mg/kg, s.c.) and spiperone (0.5-5 mg/kg, s.c.) (5-HT2A receptor antagonists), as well as a bolus of dl-p-chlorophenylalanine methyl ester (PCPA, 1000 mg/kg, p.o., 1, 6 or 24 h before administration of the sample) (an inhibitor of 5-HT synthesizing enzyme tryptophan hydroxylase) and reserpine (5 mg/kg, p.o.) (a 5-HT depletor), but not 6-hydroxydopamine (80 mg/kg, i.p., a dopamine depletor) or repeated PCPA (300 mg/kg x2, p.o., 72 and 48 h before administration of the sample), also attenuated the effects of Escin IB.
CONCLUSIONS:
It is postulated that Escin IB accelerates GIT, at least in part, by stimulating the synthesis of 5-HT to act through 5-HT2, possibly 5-HT2A receptors, which in turn causes the release of NO and PGs.
HPLC of Escin IB
