
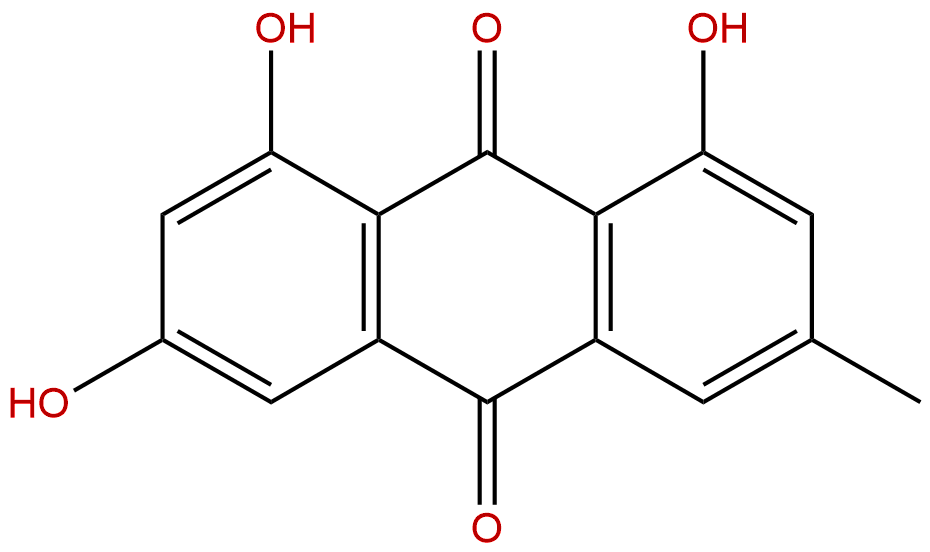
EmodinCAS No.:518-82-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0532 |
| Formula: | C15H10O5 |
| Mol Weight: | 270.24 |
Product name: Emodin
Synonym name: Rheum-emodin; Frangula emodin; Archin; Frangulinic acid; Emodol; Alatinone
Catalogue No.: BP0532
Cas No.: 518-82-1
Formula: C15H10O5
Mol Weight: 270.24
Botanical Source: Present in Cascara sagrada (Rhamnus purshiana), in aloes Aloe spp., Rumex, Polygonum. and other plants. Penicillium spp., Aspergillus spp., Dermocybe sanguinea and Anixiella micropetrusa. First isol. in 1858
Physical Description: Yellow powder
Type of Compound: Anthraquinones
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Emodin, a natural anthraquinone derivative isolated from Rheum palmatum L., exhibits anti-cancer effect on several human cancers such as liver cancers and lung cancers, treatment with 50 microM emodin resulted in a pronounced release of cytochrome c, activation of caspase-2, -3, and -9, and apoptosis in human lung adenocarcinoma A549 cells.[1]
Emodin has a strong antimicrobial activity and anti-virus effect , can inhibit HBV DNA replication and HBsAg secretion in HepG2.2.15 cells, may be a new modality to treat hepatitis B infection. [2]
Emodin significantly inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced in vitro invasion of human cancer cells including HSC5 and MDA-MB-231 cells, and inhibits the invasiveness of human cancer cells by suppressing MMP-9 expression through inhibiting AP-1 and NF-kappaB signaling pathways.[3]
References:
[1] Su Y T, Chang H L, Shyue S K, et al. Biochem Pharmacol, 2005, 70(2):229–241.
[2] Shuangsuo D, Zhengguo Z, Yunru C, et al. 2006, 12(9):302-6.
[3] Huang Q, Shen H M, Ong C N. Biochem Pharmacol, 2004, 68(2):361-71.
[4] Shi Y B, Shi Y P, Yang Y B, et al. Chromatographia, 2007, 65(9-10):601-606.
HPLC of Emodin

HNMR of Emodin
