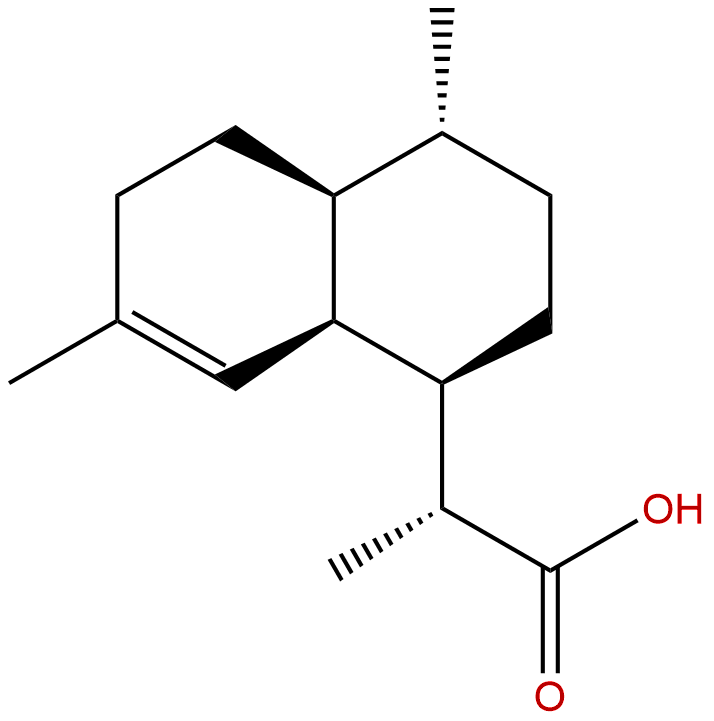
Dihydroartemisinic acidCAS No.:85031-59-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3702 |
| Formula: | C15H24O2 |
| Mol Weight: | 236.355 |
Product Name: Dihydroartemisinic acid
Synonym name: Dihydro-Artmisinic Acid
Catalogue No.: BP3702
Cas No.: 85031-59-0
Formula: C15H24O2
Mol Weight: 236.355
Botanical Source: Artemisia annua
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams. Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Dihydroartemisinic acid is a precursor to the antimalarial agent artemisinin.
References:
Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):E111-8.
Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin.
Malaria, caused by Plasmodium sp, results in almost one million deaths and over 200 million new infections annually. The World Health Organization has recommended that artemisinin-based combination therapies be used for treatment of malaria. Artemisinin is a sesquiterpene lactone isolated from the plant Artemisia annua. However, the supply and price of artemisinin fluctuate greatly, and an alternative production method would be valuable to increase availability.
METHODS AND RESULTS:
We describe progress toward the goal of developing a supply of semisynthetic artemisinin based on production of the artemisinin precursor amorpha-4,11-diene by fermentation from engineered Saccharomyces cerevisiae, and its chemical conversion to Dihydroartemisinic acid, which can be subsequently converted to artemisinin. Previous efforts to produce artemisinin precursors used S. cerevisiae S288C overexpressing selected genes of the mevalonate pathway [Ro et al. (2006) Nature 440:940-943]. We have now overexpressed every enzyme of the mevalonate pathway to ERG20 in S. cerevisiae CEN.PK2, and compared production to CEN.PK2 engineered identically to the previously engineered S288C strain. Overexpressing every enzyme of the mevalonate pathway doubled artemisinic acid production, however, amorpha-4,11-diene production was 10-fold higher than artemisinic acid. We therefore focused on amorpha-4,11-diene production. Development of fermentation processes for the reengineered CEN.PK2 amorpha-4,11-diene strain led to production of > 40 g/L product.
CONCLUSIONS:
A chemical process was developed to convert amorpha-4,11-diene to Dihydroartemisinic acid, which could subsequently be converted to artemisinin. The strains and procedures described represent a complete process for production of semisynthetic artemisinin.
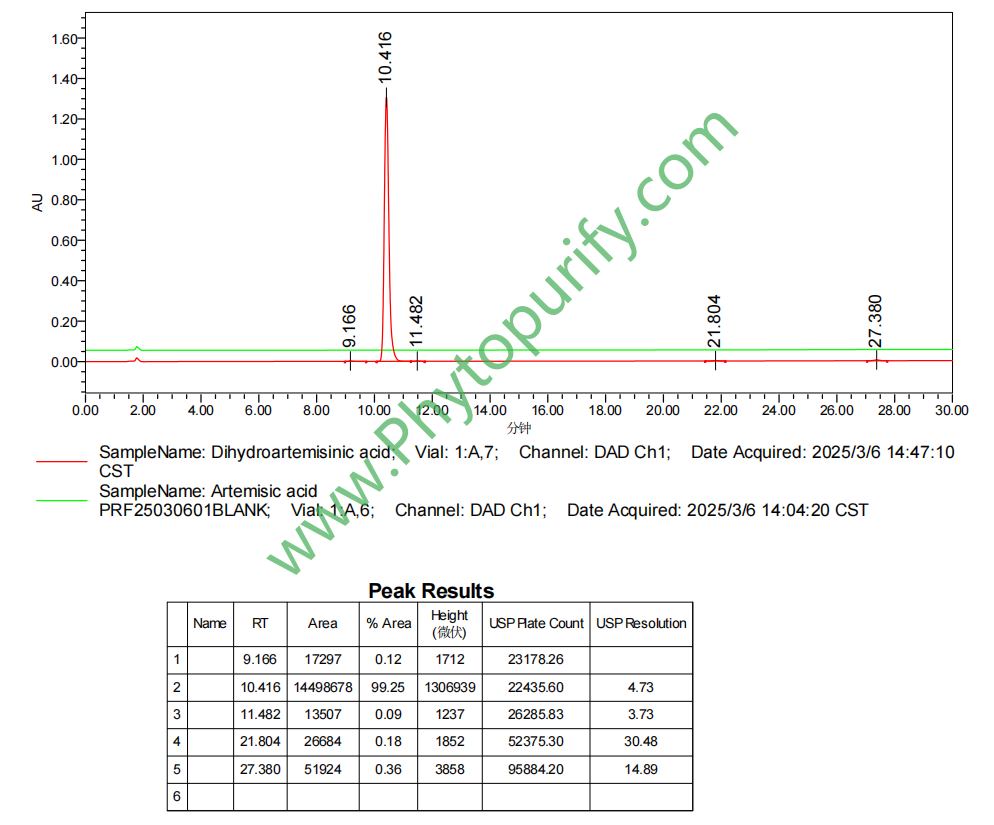
HPLC of Dihydroartemisinic acid