
DigoxinCAS No.:20830-75-5
|
||||||||||
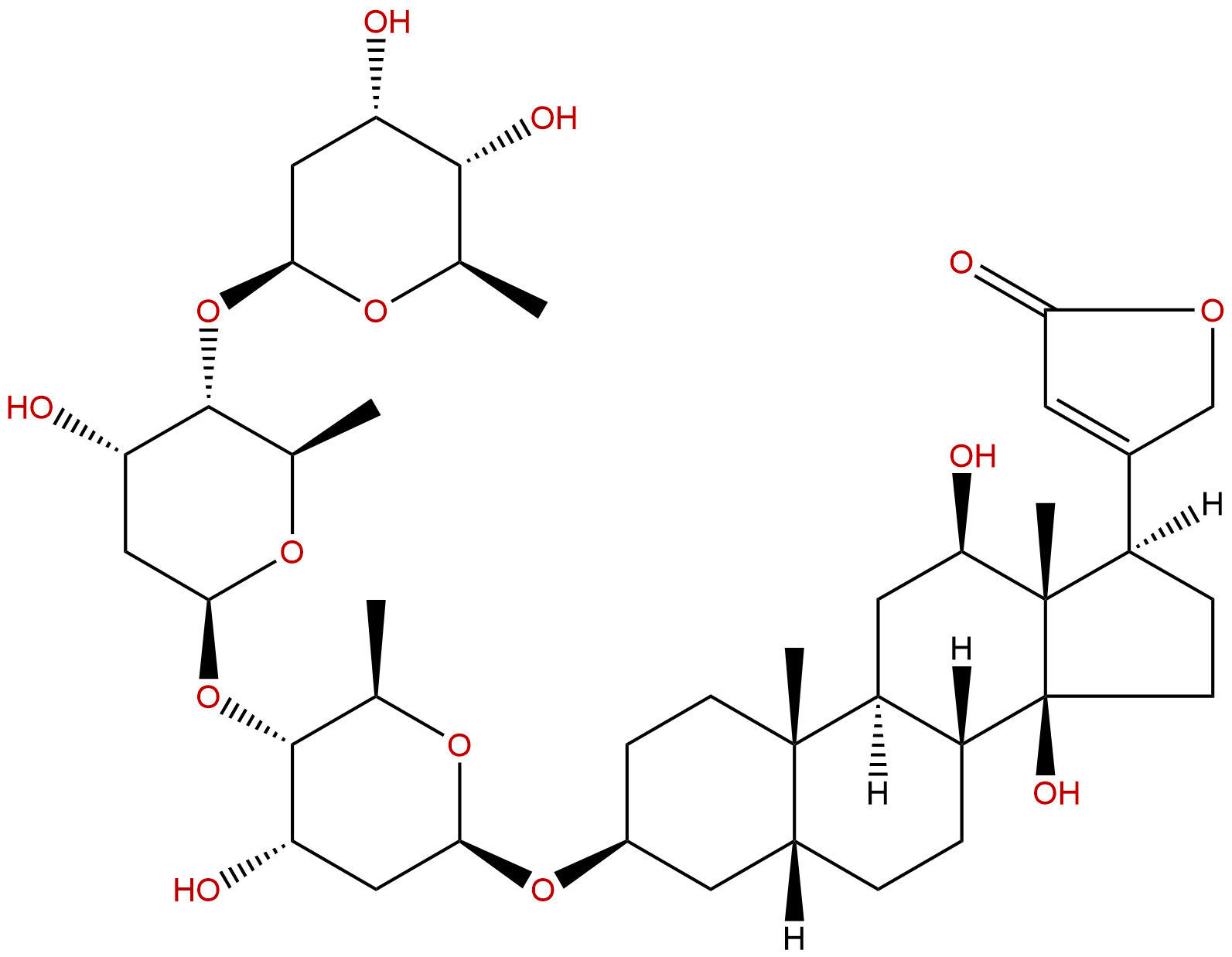 |
|
|
||||||||

| Catalogue No.: | BP0490 |
| Formula: | C41H64O14 |
| Mol Weight: | 780.949 |
Synonym name: Davoxin; Digacin; Dilanacin; Lanoxin; Rougoxin; Digosin; Cordioxil
Catalogue No.: BP0490
Cas No.: 20830-75-5
Formula: C41H64O14
Mol Weight: 780.949
Botanical Source: Digitalis lanata
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Digoxin is a classical Na,K-ATPase inhibitor, with selectivity for the α2β3 isoform over the common α1β1 isoform, used in the treatment of atrial fibrillation and heart failure. Digoxin and other cardiac glycosides can inhibit hypoxia-inducible factor 1 (HIF-1)α synthesis and block tumor growth.
References:
Lancet. 2015 Jun 13;385(9985):2363-70.
Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in
Digoxin is a widely used drug for ventricular rate control in patients with atrial fibrillation (AF), despite a scarcity of randomised trial data.
METHODS AND RESULTS:
We studied the use and outcomes of Digoxin in patients in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). For this retrospective analysis, we included and classified patients from ROCKET AF on the basis of Digoxin use at baseline and during the study. Patients in ROCKET AF were recruited from 45 countries and had AF and risk factors putting them at moderate-to-high risk of stroke, with or without heart failure. We used Cox proportional hazards regression models adjusted for baseline characteristics and drugs to investigate the association of Digoxin with all-cause mortality, vascular death, and sudden death. ROCKET AF was registered with ClinicalTrials.gov, number NCT00403767. Digoxin treatment was associated with a significant increase in all-cause mortality, vascular death, and sudden death in patients with AF.
CONCLUSIONS:
This association was independent of other measured prognostic factors, and although residual confounding could account for these results, these data show the possibility of Digoxin having these effects. A randomised trial of Digoxin in treatment of AF patients with and without heart failure is needed.
Circ Arrhythm Electrophysiol. 2015 Feb;8(1):49-58.
Digoxin and risk of death in adults with atrial fibrillation: the ATRIA-CVRN study.
Digoxin remains commonly used for rate control in atrial fibrillation, but limited data exist supporting this practice and some studies have shown an association with adverse outcomes. We examined the independent association between Digoxin and risks of death and hospitalization in adults with incident atrial fibrillation and no heart failure.
METHODS AND RESULTS:
We performed a retrospective cohort study of 14,787 age, sex, and high-dimensional propensity score-matched adults with incident atrial fibrillation and no previous heart failure or Digoxin use in the AnTicoagulation and Risk factors In Atrial fibrillation-Cardiovascular Research Network (ATRIA-CVRN) study within Kaiser Permanente Northern and Southern California. We examined the independent association between newly initiated Digoxin and the risks of death and hospitalization using extended Cox regression. During a median 1.17 (interquartile range, 0.49-1.97) years of follow-up among matched patients with atrial fibrillation, incident Digoxin use was associated with higher rates of death (8.3 versus 4.9 per 100 person-years; P<0.001) and hospitalization (60.1 versus 37.2 per 100 person-years; P<0.001). Incident Digoxin use was independently associated with a 71% higher risk of death (hazard ratio, 1.71; 95% confidence interval, 1.52-1.93) and a 63% higher risk of hospitalization (hazard ratio, 1.63; 95% confidence interval, 1.56-1.71). Results were consistent in subgroups of age and sex and when using intent-to-treat or on-treatment analytic approaches.
CONCLUSIONS:
In adults with atrial fibrillation, Digoxin use was independently associated with higher risks of death and hospitalization. Given other available rate control options, Digoxin should be used with caution in the management of atrial fibrillation.