DidyminCAS No.:14259-47-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1502 |
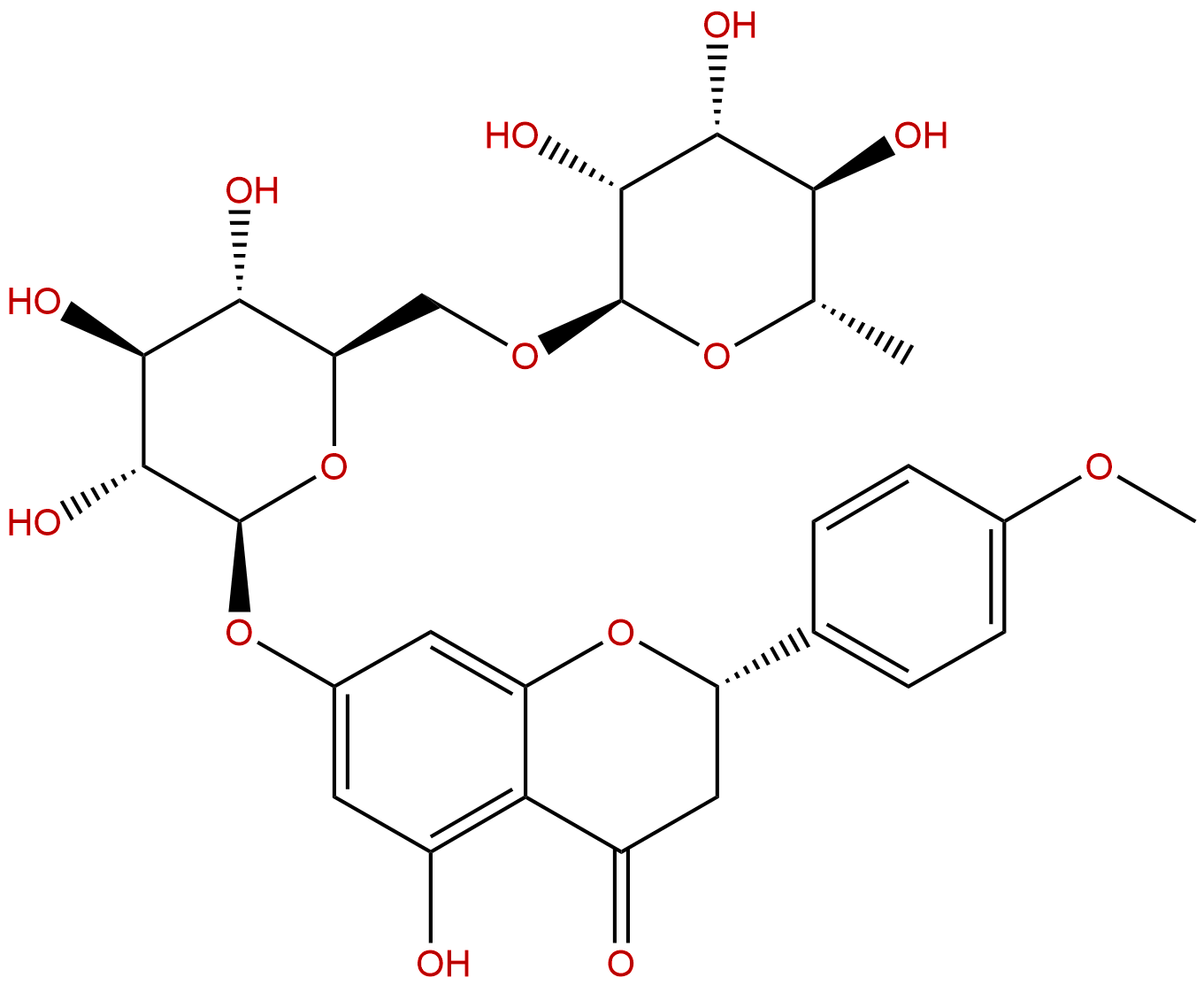
| Formula: | C28H34O14 |
| Mol Weight: | 594.566 |
Product name: Didymin
Synonym name: Neoponcirin; (S)-5,7-Dihydroxy-4′-methoxyflavanone-7-β-rutinoside;Isosakuranetin 7-O-rutinoside
Catalogue No.: BP1502
Cas No.: 14259-47-3
Formula: C28H34O14
Mol Weight: 594.566
Botanical Source: "Leaves of Monarda didyma (bergamot), from Acinos alpinus and Citrus spp."
Physical Description: Powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Didymin is a citrus-derived natural compound that kills p53 wild-type as well as drug-resistant p53-mutant neuroblastoma cells in culture, it induces apoptosis by inhibiting N-Myc and upregulating RKIP in neuroblastoma. Didymin possesses antioxidant, anti-inflammation and anti-cancer properties.
References:
Cells Tissues Organs. 2014;199(2-3):184-200.
Neuroprotective effect of didymin on hydrogen peroxide-induced injury in the neuronal membrane system.
METHODS AND RESULTS:
In this study, the flavonoid Didymin was administered in vitro in neuronal cells after hydrogen peroxide (H2O2)-induced injury (neurorescue) in order to investigate the effects of this natural molecule on cell damage in a neuronal membrane system. The results showed the effects of Didymin in neuronal cells by using a polycaprolactone biodegradable membrane system as an in vitro model. Two major findings are presented in this study: first is the antioxidant property of Didymin and, second, for the first time we provide evidence concerning its ability to rescue neuronal cells from oxidative damage. Didymin showed radical scavenging activities and it protected the neuronal cells against H2O2-induced neurotoxicity. Didymin increased cell viability, decreased intracellular reactive oxygen species generation, stimulated superoxide dismutase, catalase and glutathione peroxidase activity in neuronal cells which were previously insulted with H2O2. In addition, Didymin strikingly inhibited H2O2-induced mitochondrial dysfunctions in terms of reduction of mitochondria membrane potential and the activation of cleaved caspase-3, and also decreased dramatically the H2O2-induced phosphorylation of c-Jun N-terminal kinase. Therefore, this molecule is capable of inducing recovery from oxidative damage, and promoting and/or restoring normal cellular conditions. Moreover, the mechanism underlying the protective effects of Didymin in H2O2-injured neuronal cells might be related to the activation of antioxidant defense enzymes as well as to the inhibition of apoptotic features, such as p-JNK and caspase-3 activation.
CONCLUSIONS:
These data suggest that Didymin may be a potential therapeutic molecule for the treatment of neurodegenerative disorders associated with oxidative stress.
Lung Cancer. 2010 Jun;68(3):366-74.
Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo.
Epidemiological studies provided evidence that the high dietary intake of flavonoids with fruits and vegetables could be associated with lower cancer prevalence in humans. Didymin, a dietary flavonoid glycoside from citrus fruits, possesses antioxidant properties.
METHODS AND RESULTS:
This study first investigates the anticancer effect of Didymin in human non-small-cell lung cancer A549 and H460 cells. To identity the anticancer mechanism of Didymin, we assayed its effect on apoptosis, cell cycle distribution, and levels of p53, p21/WAF1, Fas/APO-1 receptor, and Fas ligand. The results showed that Didymin-induced apoptosis of A549 and H460 cells without mediation of p53 and p21/WAF1. We suggest that Fas/Fas ligand apoptotic system is the main pathway of Didymin-mediated apoptosis of A549 and H460 cells. Importantly, a novel chemotherapeutic agent for the treatment of non-small-cell lung cancer, and is supported by animal studies which have shown Didymin delay the tumor growth in nude mice.
CONCLUSIONS:
Our study reports here for the first time that the activity of the Fas/Fas ligand apoptotic system may participate in the antiproliferative activity of Didymin in A549 and H460 cells.
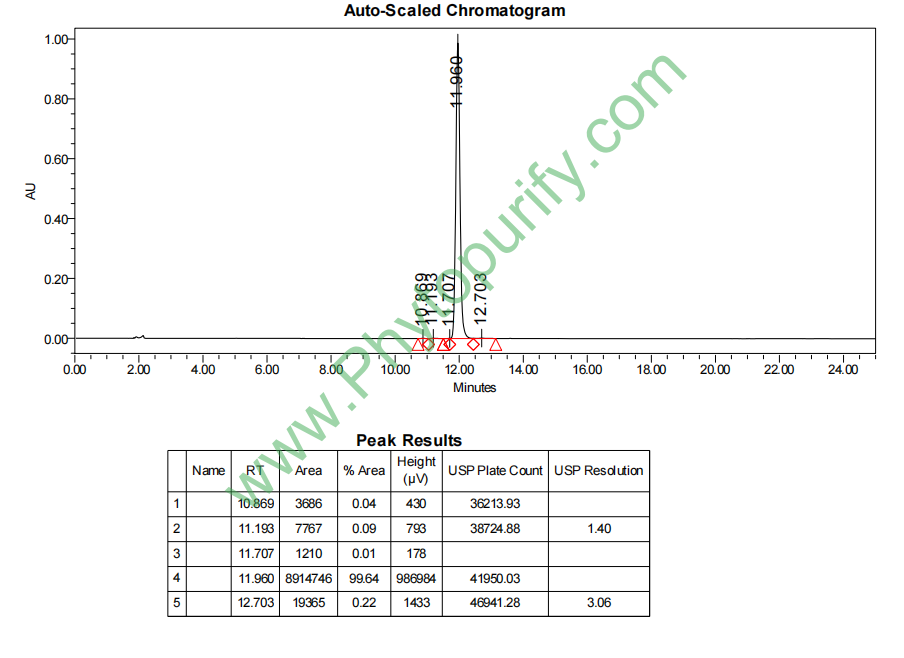
HPLC of Didymin