
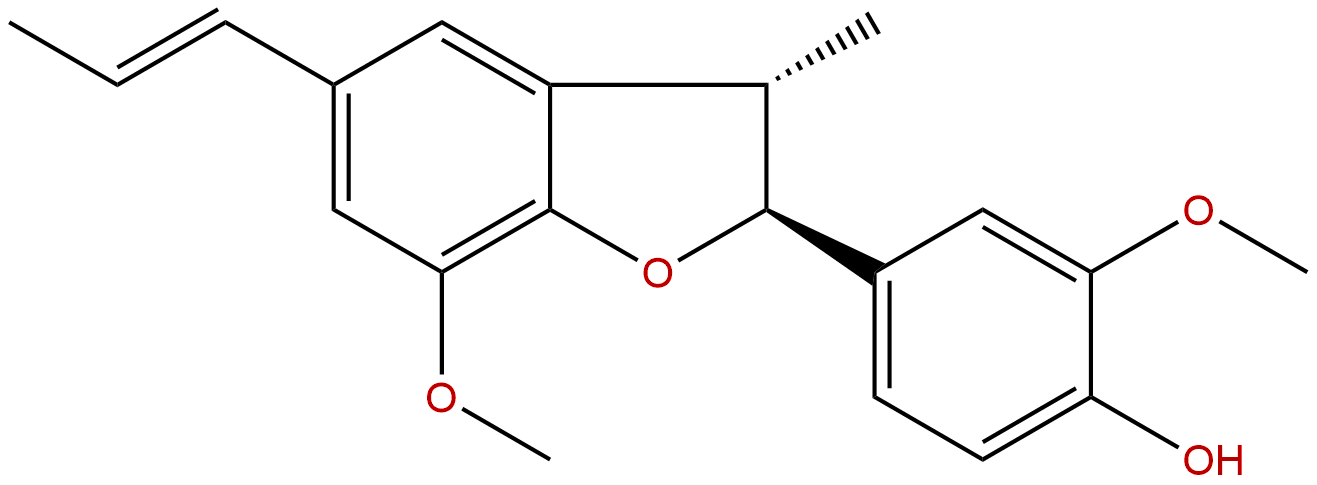
DehydrodiisoeugenolCAS No.:2680-81-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0463 |
| Formula: | C20H22O4 |
| Mol Weight: | 326.392 |
Synonym name: 83377-50-8
Catalogue No.: BP0463
Cas No.: 2680-81-1
Formula: C20H22O4
Mol Weight: 326.392
Botanical Source: Aristolochia pubescens, Krameria grayi, Myristica fragrans (mace) and Piper kadsura
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Dehydrodiisoeugenol has anti-inflammatory activity, it inhibited the expression of the COX-2, proteolysis of inhibitor κB-α and transcriptional activity of NF-κB. Dehydrodiisoeugenol can cross the blood-brain barrier rapidly, it may be developed into an effective anxiogenic agent.
References:
Planta Med. 2011 Oct;77(15):1712-7.
Metabolism of the lignan dehydrodiisoeugenol in rats.
Dehydrodiisoeugenol (DDIE), a major active lignan from the seed and aril of the fruit of Myristica fragrans Houtt., functions as a potential anti-inflammatory agent by inhibiting lipopolysaccharide-stimulated nuclear factor kappa B activation and cyclooxygenase-2 expression in macrophages. However, the metabolism of DDIE remains unknown.
METHODS AND RESULTS:
This report describes the metabolic fate of DDIE in liver microsomes, urine, and feces of rats treated with DDIE. DDIE metabolites were isolated by sequential column chromatography and high-performance liquid chromatography from liver microsomes incubations, urine, and feces. Nine metabolites ( M-1 to M-9), including 5 new metabolites, were determined spectroscopically using ultra-violet (UV), mass spectrometry (MS), nuclear magnetic resonance (NMR), and circular dichroism (CD). Analysis of the isolated metabolites showed that DDIE undergoes four major pathways of metabolism in the rat: oxidation (including hydroxylation, hydroformylation, and acetylation), demethylation, ring-opening, and dehydrogenation. In contrast to the metabolites from liver microsomes, the major metabolites In vivo were generated from DDIE by multiple metabolic reactions.
CONCLUSIONS:
Given these results, we describe a metabolic pathway for DDIE in the rat that gives insight into the metabolism of DDIE and the mechanism of DDIE bioactivity in humans.