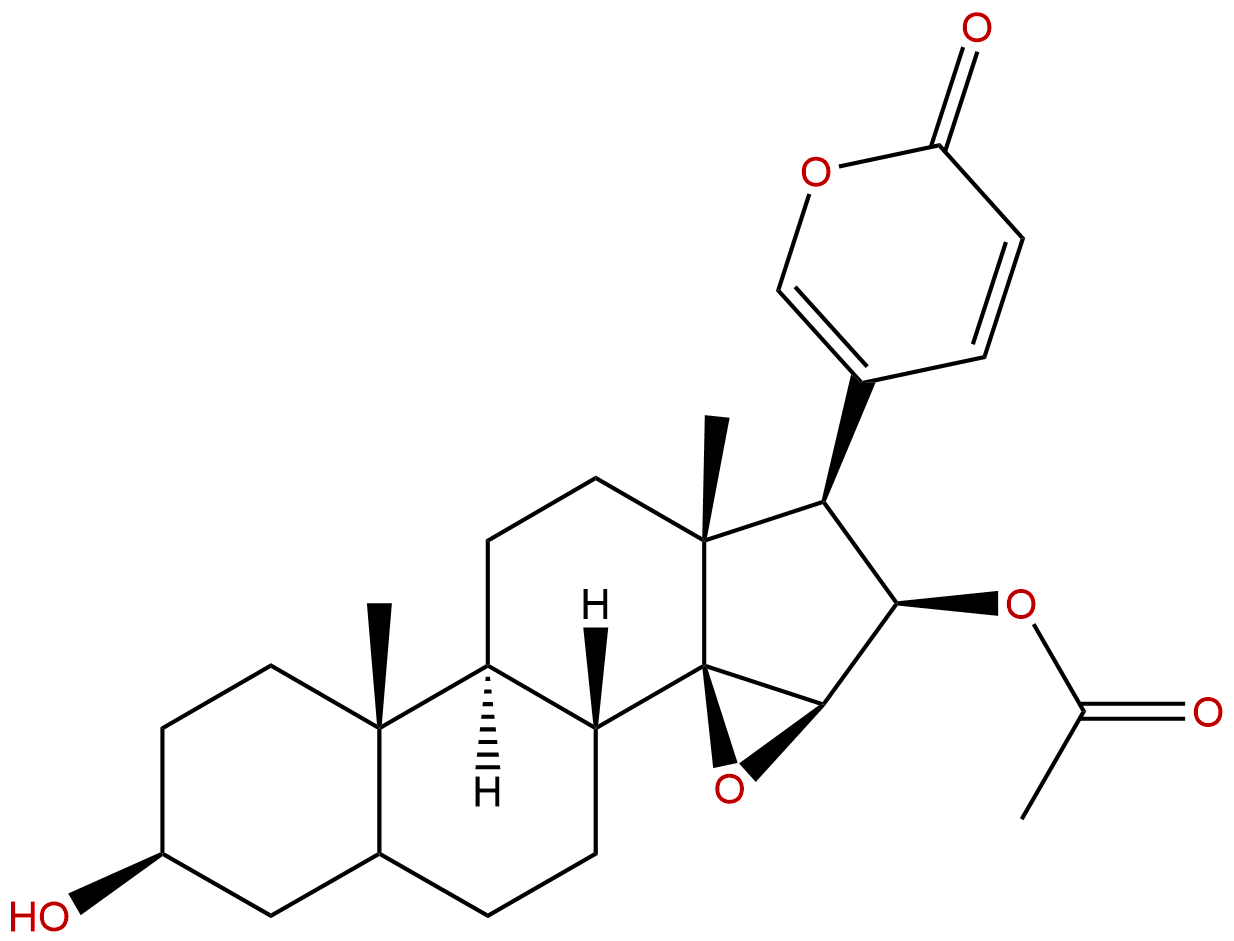
Cinobufagin Descrtption
Product name: Cinobufagin
Synonym name: Cinobufagine
Catalogue No.: BP0366
Cas No.: 470-37-1
Formula: C26H34O6
Mol Weight: 442.552
Botanical Source: Bufonis Venenum
Physical Description:
Type of Compound: Steroids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Cinobufagin, a kind of Chinese materia medica with antitumor effect, is widely used in clinical practice, especially in anti-liver cancer, it also has anti-hepatitis B virus activity . Cinobufagin inhibits the proliferation and induces apoptosis, may be related to the mitochondria-mediated pathway and GSK-3β/NF-κB pathway. Cinobufagin can significantly relieve cancer pain in mice and raised their pain threshold, mainly upregulating the expression levels of β -END and μ -OR in the hind paw tumor and adjacent tissue.Cinobufagin and bufalin exhibit cardiotonic and natriuretic activities, they also have inhibitory effects on steroidogenesis of aldosterone and cortisol.
References:
Immunopharmacol Immunotoxicol. 2015 May 18:1-9.Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway.
Cinobufagin (CBG) is a cardiotoxic bufanolide steroid secreted by the skin and parotid venom glands of the Asiatic toad Bufo bufo gargarizans (called Chan-Su). Although CBG is known to exhibit anti-cancer activities, very little is known about its potential mechanism(s) of action. In this study, we investigated whether CBG mediates its effect through the modulation of the mitogen-activated protein kinases (MAPKs) signaling pathway in human multiple myeloma (MM) U266 cells.
METHODS AND RESULTS:We found that CBG caused the significant activation of ERK, JNK and p38 MAPK in U266 cells. CBG showed much higher cytotoxicity against U266 cells as compared to peripheral blood mononuclear cells (PBMC). Induction of CBG increased reactive oxygen species (ROS) generation from mitochondria, which is associated with the induction of apoptosis as characterized by increased sub-G1 DNA contents of cell cycle, positive Annexin V binding, activation of caspase-3 and cleavage of PARP. Inhibition of ROS generation by N-acetyl-l-cysteine (NAC) significantly prevented CBG-induced ERK, JNK and p38 MAPK activation and apoptosis. CBG also down-regulated the expression of various downstream gene products that mediate cell proliferation, survival, angiogenesis and metastasis. Interestingly, ERK, JNK and p38MAPK pharmacological inhibitors blocked CBG-induced MAPKs activation and ERK inhibitor (PD98059) also prevented the CBG-induced caspase-3 activation and PARP cleavage in U266 cells.
METHODS AND RESULTS:Taken together, these findings suggest that CBG can act as a potent anticancer agent against MM and possibly exerts its effects through the ROS-mediated activation of ERK, JNK and p38 MAPK leading to the activation of caspase-3 in U266 cells.
Biol Pharm Bull. 2010;33(10):1728-32.Anti-hepatitis B virus activities of cinobufacini and its active components bufalin and cinobufagin in HepG2.2.15 cells.
Cinobufacini (Huachansu) is a Chinese medicine prepared from the skin of Bufo bufo gargarizans Cantor (Bufonidae), which has long been used in traditional Chinese medicine (TCM). The aim of present study was to examine the anti-hepatitis B virus (HBV) activities of cinobufacini and its active components bufalin and Cinobufagin in the human HBV-transfected cell line HepG2.2.15.
METHODS AND RESULTS:The hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core-related antigen (HBcrAg) concentrations in cell culture medium were determined by chemiluminescent enzyme immunoassay after HepG2.2.15 cells were respectively treated with different concentrations of cinobufacini, bufalin, and Cinobufagin for 3 or 6 d. HBV DNA and mRNA were determined using transcription-mediated amplification and real-time polymerase chain reaction (PCR), respectively. On d 3, cinobufacini at a concentration of 1 μg/ml had no activity against HBV virological markers. However, on d 6, cinobufacini at 1 μg/ml effectively inhibited the secretion of HBsAg, HBeAg, and HBcrAg by 29.58, 32.87, and 42.52%. It was more potent than the positive control lamivudine (100 μg/ml). Bufalin and Cinobufagin slightly inhibited HBV antigen secretion. Treatment with cinobufacini, bufalin, or Cinobufagin had no anti-HBV effect on DNA in cell culture medium. Consistent with the HBV antigen reduction, HBV mRNA expression was markedly inhibited in comparison to the control when HepG2.2.15 cells were treated with cinobufacini, bufalin, or Cinobufagin.
CONCLUSIONS:Results suggested that cinobufacini had more potent activity against HBV antigen secretion than its components bufalin and Cinobufagin and this inhibitory role was attributed to the specific inhibition of HBV mRNA expression.
Evid Based Complement Alternat Med. 2013;2013:851256.
A Study on the Mechanism of Cinobufagin in the Treatment of Paw Cancer Pain by Modulating Local β -Endorphin Expression In Vivo.
Cinobufagin has been widely used in the treatment of carcinoma and plays an important role in the relief of cancer pain. But the involved mechanism remains unknown. To investigate the changes in thermal and mechanical hyperalgesia in paw cancer pain in mice and the action mechanism of Cinobufagin using a paw cancer pain model.
METHODS AND RESULTS:
60 female mice were randomly divided into 5 groups: control group, model group, Cinobufagin group, Cinobufagin +NAL-M group, and morphine group; except ones in control group, mice were inoculated with H22 hepatoma cells in the right hind paw. From the 9th day after inoculation, mice were administrated drug once daily lasting for 8 days. The pain behavior was determined on the 2nd, 4th, 6th, and 8th days before and after administration. On the last day, they were sacrificed. The levels of β -END, CRF, and IL-1 β were analyzed by ELISA; immunohistochemistry was performed to detect the expressions of β -END, POMC, and μ -OR in the tumor and adjacent tissue. The thresholds of thermal pain and mechanical pain were significantly increased by Cinobufagin. Moreover, the expressions of β -END, CRF, POMC, and μ -OR were significantly upregulated by Cinobufagin. The analgesic effect of Cinobufagin was blocked by the peripheral opioid receptor antagonist NAL-M.
METHODS AND RESULTS:
Cinobufagin significantly relieved cancer pain in mice and raised their pain threshold, mainly upregulating the expression levels of β -END and μ -OR in the hind paw tumor and adjacent tissue.
HPLC of Cinobufagin