
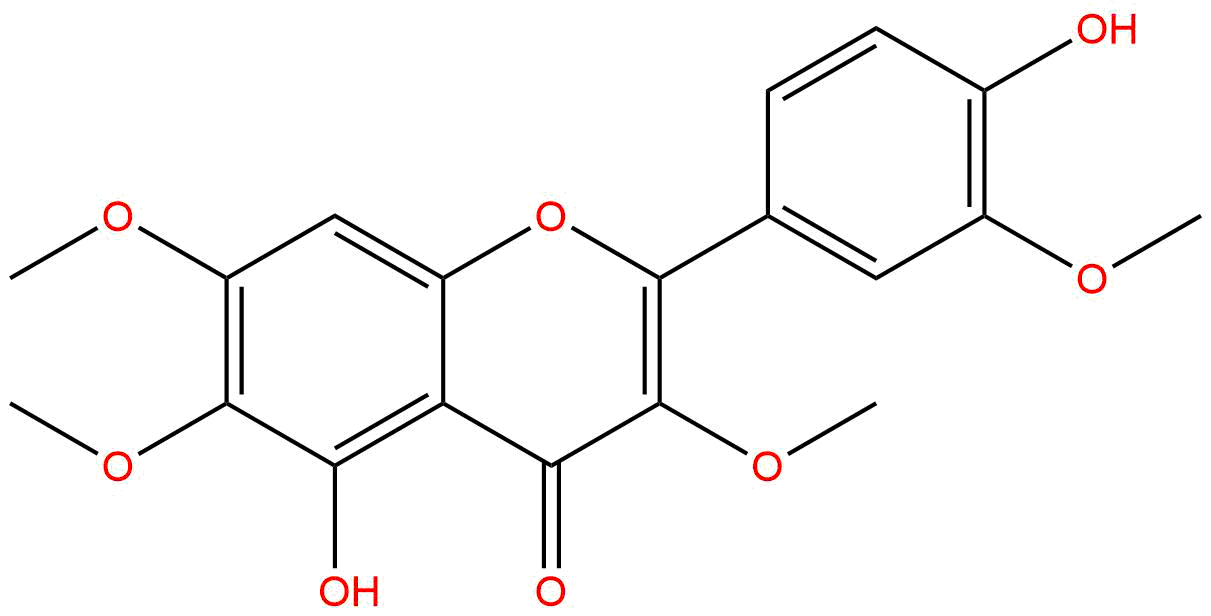
Chrysosplenetin BCAS No.:603-56-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | SBP00206 |
| Formula: | C19H18O8 |
| Mol Weight: | 374.345 |
Product name: Chrysosplenetin B
Synonym name: Chrysosplenetin
Catalogue No.: SBP00206
Cas No.: 603-56-5
Formula: C19H18O8
Mol Weight: 374.345
Botanical Source:
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Chrysosplenetin is a metabolic inhibitor of artemisinin, it has strong activity in vitro against EV71 with low cytotoxicity. Co-administration of artemisinin(ART) with chrysosplenetin(CHR) in ratio of 1:2 achieved a synergic anti-malarial effect partly because of the noncompetitive or uncompetitive inhibition of CHR of drug-metabolism enzymes, especially CYP3A which is closely related to the auto-induction of ART.
References:
Zhongguo Zhong Yao Za Zhi. 2013 Oct;38(19):3363-7.
Determination of chrysosplenetin, metabolic inhibitor of artemisinin, in rat plasma by UPLC-ms/MS and study on its pharmacokinetics.
METHODS AND RESULTS:
The study aimed to develop the assay of Chrysosplenetin (CHR), a metabolic inhibitor of artemisinin by UPLC-MS/MS in rat plasma and investigate the pharmacokinetics parameters of Chrysosplenetin.The assay was linear in the range 5-5 000 microg L-1 (r =0. 999 3) with recoveries in the range from 69. 0% to 81.2% and satisfied inter-, intra- precision and accuracy. Chrysosplenetin after oral administration is not easy to absorb with double or multimodal peak phenomenon. The t1/2 of Chrysosplenetin after intravenous injection was very short and that of low, medium, and high dosage was (17. 01 +/- 8. 06) , (24. 62 +/- 4. 59), (28. 46+/- 4. 63) min, respectively.
CONCLUSIONS:
The developed method was special, rapid, and sensitive for determination of Chrysosplenetin pharmacokinetics.
Eur J Pharm Sci. 2011 Oct 9;44(3):392-8.
Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin.
In recent years, enterovirus 71 (EV71) infections have caused an increasing epidemic in young children, accompanying with more severe nervous system disease and more deaths. Unfortunately, there is no specific medication for it so far.
METHODS AND RESULTS:
Here we investigated the anti-EV71 activity of Chrysosplenetin and penduletin, two o-methylated flavonols isolated from the leaves of Laggera pterodonta. These two compounds were found to have strong activity in vitro against EV71 with low cytotoxicity. In the cytopathic effect (CPE) inhibition assays, both plaque reduction assay and virus yield inhibition assay, the compounds showed a similar 50% inhibitory concentration (IC(50)) value of about 0.20 μM.
CONCLUSIONS:
The selectivity indices (SI) of Chrysosplenetin and penduletin were 107.5 and 655.6 in African green monkey kidney (Vero) cells, and 69.5 and 200.5 in human rhabdomyosarcoma (RD) cells, accordingly.
Malaria J., 2015, 14(1):1-13.
Impact of chrysosplenetin on the pharmacokinetics and anti-malarial efficacy of artemisinin against Plasmodium berghei as well as in vitro CYP450 enzymatic activities in rat liver microsome.
Artemisinin (ART) is an efficacious and safe anti-malarial drugs but has low oral bioavailability and auto-induction profiles during multiple dosing. The pharmacokinetic disadvantages have been found to partially depend on the induction of cytochrome P-450 enzymes by ART and resulted in the therapeutic failure due to insufficient drug levels.
METHODS AND RESULTS:
The present study, therefore, investigated the impacts of Chrysosplenetin (CHR), a polymethoxylated flavonoid from Artemisia annua, on the pharmacokinetics and the anti-malarial efficacy of ART against Plasmodium berghei. The inhibition of CHR on enzymatic activity of CYP1A2, CYP2A, CYP2C19, CYP2D6, CYP2E1, and CYP3A in rat liver microsome was also investigated. IC50, Km, Ki, and inhibitory type of CHR were respectively calculated. Co-administration of ART with CHR in ratio of 1:2 achieved a synergic anti-malarial effect partly because of the noncompetitive or uncompetitive inhibition of CHR of drug-metabolism enzymes, especially CYP3A which is closely related to the auto-induction of ART.