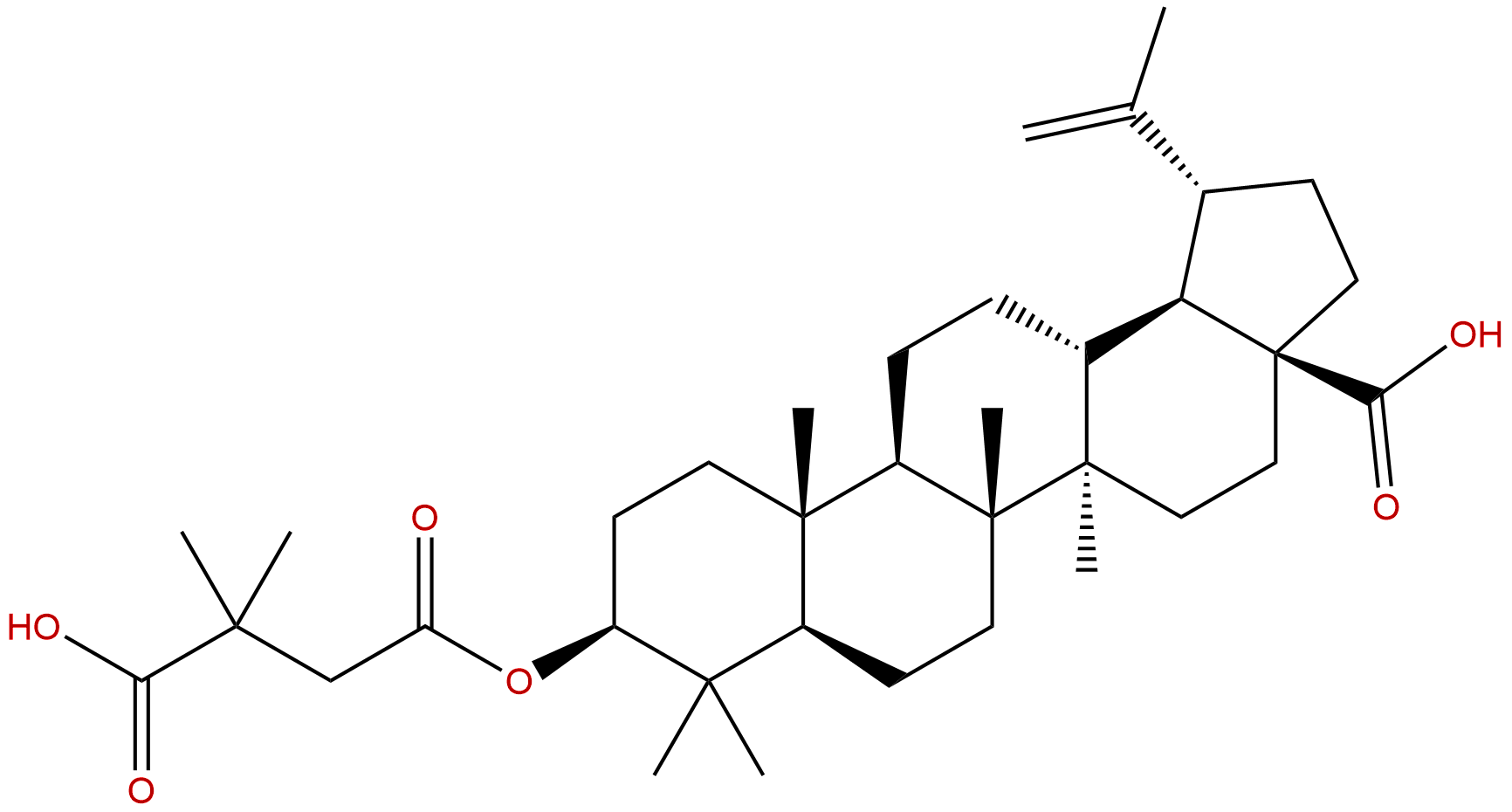
BevirimatCAS No.:174022-42-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3086 |
| Formula: | C36H56O6 |
| Mol Weight: | 584.838 |
Product name: Bevirimat
Synonym name: PA-457
Catalogue No.: BP3086
Cas No.: 174022-42-5
Formula: C36H56O6
Mol Weight: 584.838
Botanical Source:
Physical Description:
Type of Compound:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
Description:
Bevirimat, also known as MPC-4326 and PA-457, is an anti-HIV drug derived from a betulinic acid-like compound, first isolated from Syzygium claviflorum, a Chinese herb. It is believed to inhibit HIV by a novel mechanism, so-called maturation inhibition. Like protease inhibitors, bevirimat and other maturation inhibitors interfere with protease processing of newly translated HIV polyprotein precursor, called gag. Bevirimat prevents this viral replication by specifically inhibiting cleavage of the capsid protein (CA) from the SP1 spacer protein. (http://en.wikipedia.org/wiki/Bevirimat).
References
1: Coric P, Turcaud S, Souquet F, Briant L, Gay B, Royer J, Chazal N, Bouaziz S. Synthesis and biological evaluation of a new derivative of bevirimat that targets the Gag CA-SP1 cleavage site. Eur J Med Chem. 2013 Apr;62:453-65. doi: 10.1016/j.ejmech.2013.01.013. Epub 2013 Jan 19. PubMed PMID: 23399723.
2: Dang Z, Ho P, Zhu L, Qian K, Lee KH, Huang L, Chen CH. New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. J Med Chem. 2013 Mar 14;56(5):2029-37. doi: 10.1021/jm3016969. Epub 2013 Feb 20. PubMed PMID: 23379607; PubMed Central PMCID: PMC3600082.
3: Qian K, Bori ID, Chen CH, Huang L, Lee KH. Anti-AIDS agents 90. novel C-28 modified bevirimat analogues as potent HIV maturation inhibitors. J Med Chem. 2012 Sep 27;55(18):8128-36. Epub 2012 Sep 14. PubMed PMID: 22978745; PubMed Central PMCID: PMC3478670.
4: Dang Z, Qian K, Ho P, Zhu L, Lee KH, Huang L, Chen CH. Synthesis of betulinic acid derivatives as entry inhibitors against HIV-1 and bevirimat-resistant HIV-1 variants. Bioorg Med Chem Lett. 2012 Aug 15;22(16):5190-4. doi: 10.1016/j.bmcl.2012.06.080. Epub 2012 Jul 3. PubMed PMID: 22818973; PubMed Central PMCID: PMC3426442.
5: Nguyen AT, Feasley CL, Jackson KW, Nitz TJ, Salzwedel K, Air GM, Sakalian M. The prototype HIV-1 maturation inhibitor, bevirimat, binds to the CA-SP1 cleavage site in immature Gag particles. Retrovirology. 2011 Dec 7;8:101. doi: 10.1186/1742-4690-8-101. PubMed PMID: 22151792; PubMed Central PMCID: PMC3267693.
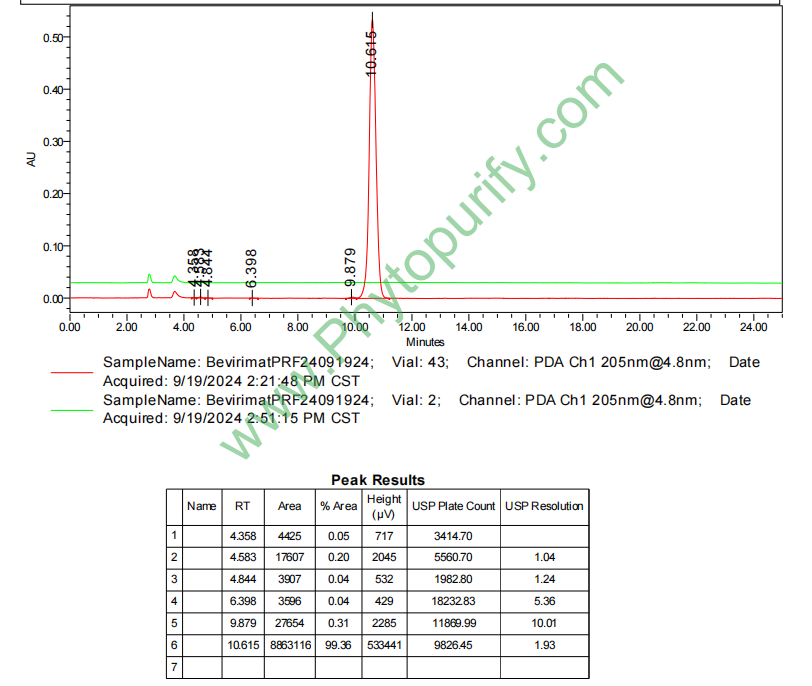
HPLC of Bevirimat