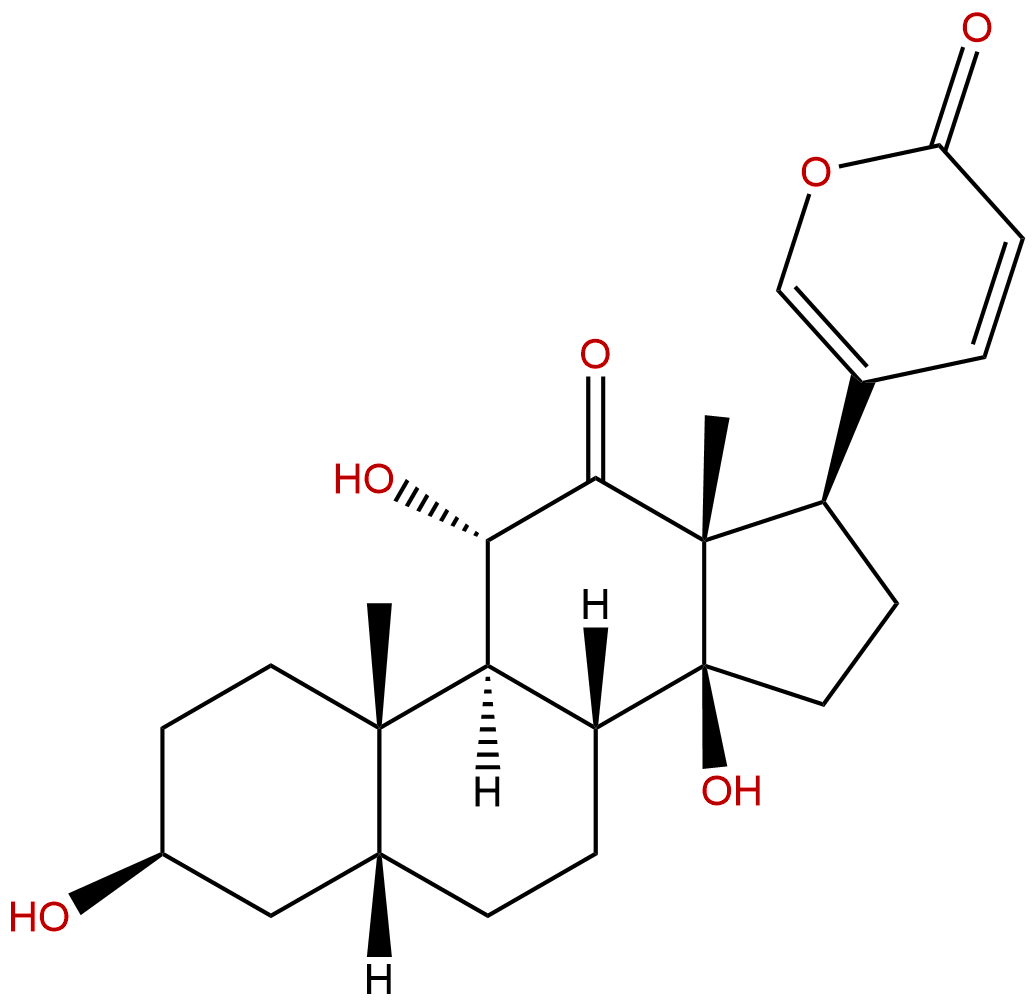
ArenobufaginCAS No.:464-74-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0190 |
| Formula: | C24H32O6 |
| Mol Weight: | 416.514 |
Synonym name: Arenobufogenin
Catalogue No.: BP0190
Cas No.: 464-74-4
Formula: C24H32O6
Mol Weight: 416.514
Botanical Source: secretion of Bufo arenarum
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Arenobufagin is a potent Na + /K + pump inhibitor, and is also a specific inhibitor of VEGF-mediated angiogenesis. Arenobufagin has antineoplastic effect that involves cross talk between apoptosis and autophagy via inhibition of the PI3K/Akt/mTOR pathway.
References:
Carcinogenesis. 2013 Jun;34(6):1331-42.
Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway.
Hepatocellular carcinoma (HCC) is a deadly form of cancer without effective chemotherapy so far. Currently, only sorafenib, a multitargeted tyrosine kinase inhibitor, slightly improves survival in HCC patients.
METHODS AND RESULTS:
In searching for natural anti-HCC components from toad venom, which is frequently used in the treatment of liver cancer in traditional Chinese medicine, we discovered that Arenobufagin, a bufadienolide from toad venom, had potent antineoplastic activity against HCC HepG2 cells as well as corresponding multidrug-resistant HepG2/ADM cells. We found that Arenobufagin induced mitochondria-mediated apoptosis in HCC cells, with decreasing mitochondrial potential, as well as increasing Bax/Bcl-2 expression ratio, Bax translocation from cytosol to mitochondria. Arenobufagin also induced autophagy in HepG2/ADM cells. Autophagy-specific inhibitors (3-methyladenine, chloroquine and bafilomycin A1) or Beclin1 and Atg 5 small interfering RNAs (siRNAs) enhanced Arenobufagin-induced apoptosis, indicating that Arenobufagin-mediated autophagy may protect HepG2/ADM cells from undergoing apoptotic cell death. In addition, we observed the inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway by Arenobufagin. Interestingly, inhibition of mTOR by rapamycin or siRNA duplexes augmented Arenobufagin-induced apoptosis and autophagy. Finally, Arenobufagin inhibited the growth of HepG2/ADM xenograft tumors, which were associated with poly (ADP-ribose) polymerase cleavage, light chain 3-II activation and mTOR inhibition.
CONCLUSIONS:
In summary, we first demonstrated the antineoplastic effect of Arenobufagin on HCC cells both in vitro and in vivo. We elucidated the underlying antineoplastic mechanisms of Arenobufagin that involve cross talk between apoptosis and autophagy via inhibition of the PI3K/Akt/mTOR pathway. This study may provide a rationale for future clinical application using Arenobufagin as a chemotherapeutic agent for HCC.
J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Nov 15;939:86-91.
Quantitative determination of arenobufagin in rat plasma by ultra fast liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study.
A rapid, sensitive, and selective ultra fast liquid chromatography-tandem mass spectrometry method was developed for quantitative determination of Arenobufagin in rat plasma.
METHODS AND RESULTS:
Sample pretreatment involved a one-step protein precipitation with methanol using 0.1mL rat plasma. The separation was carried out on a Shim-pack XR-ODS II (75mm×2.0mm, i.d. 2.1μm) column with gradient elution at a flow rate of 0.30mLmin(-1). The mobile phase was acetonitrile and 0.1% formic acid in water. A post-column switching valve was applied to reduce the matrix effect. The detection was performed on a triple-quadruple tandem mass spectrometer in the multiple reaction monitoring mode after electrospray ionization. Linear calibration curves for Arenobufagin were obtained over the concentration range 1.056-1056ngmL(-1), with a lower limit of quantification of 1.056ngmL(-1). The intra-day and inter-day precision values were lower than 15% and the accuracy ranged from 5.4% to 9.8% at all quality control levels.
CONCLUSIONS:
The method was successfully applied to the determination and pharmacokinetic study of Arenobufagin in rat plasma following intraperitoneal administration.
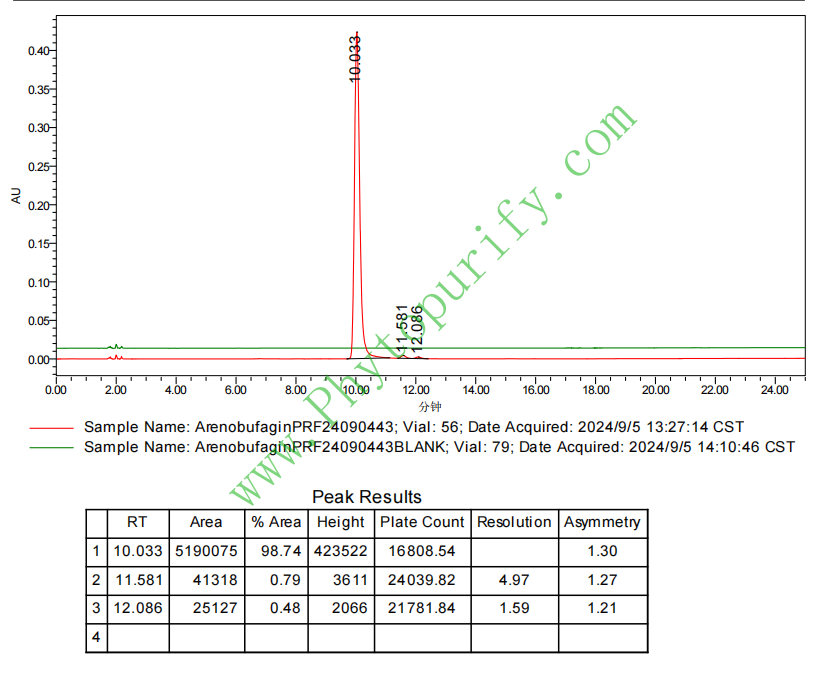
HPLC of Arenobufagin