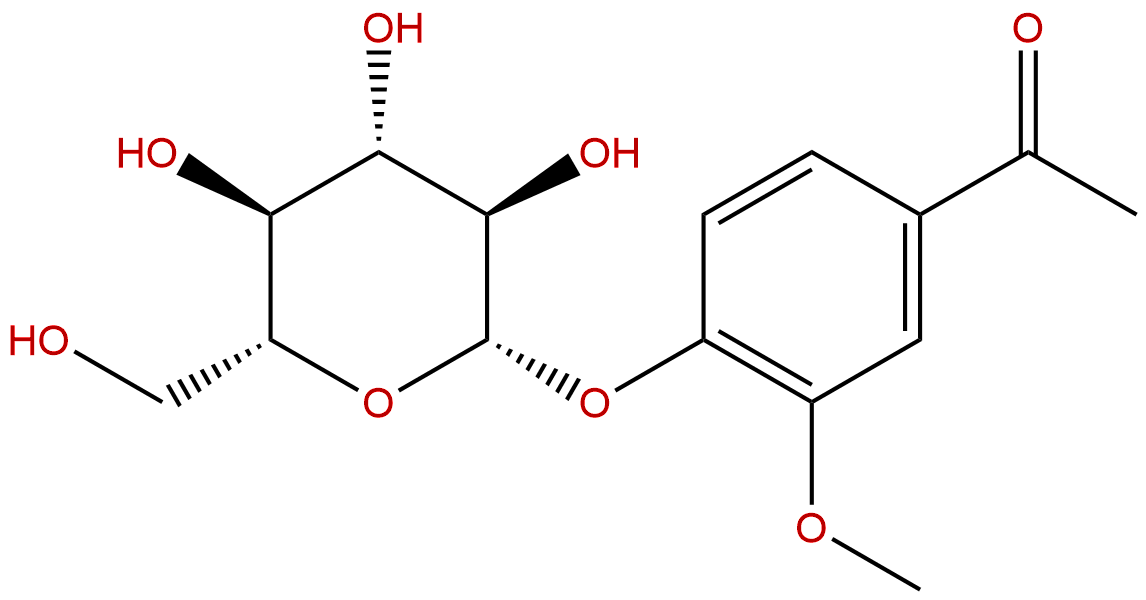
AndrosinCAS No.:531-28-2
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0165 |
| Formula: | C15H20O8 |
| Mol Weight: | 328.317 |
Product name: Androsin
Synonym name: Glucoacetovanillone
Catalogue No.: BP0165
Cas No.: 531-28-2
Formula: C15H20O8
Mol Weight: 328.317
Botanical Source: Picrorhiza scrophulariiflora Pennell
Physical Description:
Type of Compound: Miscellaneous
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Nomilin has immunomodulatory, antioxidant, anti-human immunodeficiency virus(HIV), cancer chemopreventive, antiangiogenic, anti-obesity and anti-hyperglycemic effects. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. It inhibits osteoclastogenesis in vitro by suppression of NFATc1 and MAPK signaling pathways, indicates that nomilin-containing herbal preparations have potential utility for the prevention of bone metabolic diseases.
References:
Food Chemistry, 2005, 93(4):599-605.
Contents and antioxidant capacity of limonin and nomilin in different tissues of citrus fruit of four cultivars during fruit growth and maturation.
METHODS AND RESULTS:
The contents of limonin and Nomilin in different fruit tissues of Foxiangyou (Citrus grandis), Citrus unshiu, Penggan (Citrus reticulata) and Huyou (Citrus changshanensis KS Chen et CX Fu) were measured during fruit growth and maturation by HPLC (high performance liquid chromatography). Results showed that limonin and Nomilin were the predominant limonoids in the extracted samples. During fruit growth and maturation, the contents of limonin and Nomilin increased from April, peaked in early September and decreased afterwards until late October when they reached a steady low level. The antioxidant capacities of limonin and Nomilin in the four tissues of mature fruit were determined by beta-carotene bleaching assay.
CONCLUSIONS:
The results showed that the antioxidant capacities of limonin and Nomilin varied in different tissues and cultivars. In the three tissues other than albedo, the antioxidant capacities of limonin and Nomilin were high (2.9-8.3 times than that of vitamine C).
Planta Med. 2003 Oct;69(10):910-3.
Effect of limonin and nomilin on HIV-1 replication on infected human mononuclear cells.
In the last years several plant-derived natural compounds have been screened for their anti-HIV activity in order to find lead compounds with novel structures or mechanisms of action. Among these, several triterpenoids have been found to exhibit an antiretroviral activity with different mechanisms of action.
METHODS AND RESULTS:
In this study the effect of two limonoids, limonin and Nomilin, on the growth of human immunodeficiency virus-1 (HIV-1) in culture of human peripheral blood mononuclear cells (PBMC) and on monocytes/macrophages (M/M) is described. Limonin and Nomilin were found to inhibit the HIV-1 replication in all cellular systems used. A dose-dependent inhibition of viral replication was observed in PBMC isolated from healthy donors and infected with HIV-1 strain after incubation with limonin and Nomilin (EC (50) values: 60.0 microM and 52.2 microM, respectively). The two terpenoids inhibited at all concentrations studied the production of HIV-p24 antigen even when the PBMC employed were chronically infected (EC (50) values of 61.0 microM for limonin and 76.2 microM for Nomilin). Moreover, these compounds inhibited the HIV-1 replication even in infected M/M. In this cellular system the inhibitory effect was significant at the concentrations of 20 microM, 40 microM and 80 microM starting from day 14 and reached the maximum effect after 18 days of incubation. As regards the mechanism of action, limonin and Nomilin inhibit in vitro HIV-1 protease activity.
CONCLUSIONS:
In general, the results obtained point out a similar anti-HIV activity of limonin and Nomilin indicating that this activity is not drastically influenced by the structural difference between the two compounds.
Biochem Biophys Res Commun. 2011 Jul 8;410(3):677-81.
Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet.
TGR5 is a member of the G protein-coupled receptor family and is activated by bile acids (BAs). TGR5 is thought to be a promising drug target for metabolic diseases because the activation of TGR5 prevents obesity and hyperglycemia in mice fed a high-fat diet (HFD).
METHODS AND RESULTS:
In the present study, we identified a naturally occurring limonoid, Nomilin, as an activator of TGR5. Unlike BAs, Nomilin did not exhibit the farnesoid X receptor ligand activity. Although the Nomilinderivative obacunone was capable of activating TGR5, limonin (the most abundant limonoid in citrus seeds) was not a TGR5 activator. When male C57BL/6J mice fed a HFD for 9 weeks were further fed a HFD either alone or supplemented with 0.2%w/w Nomilin for 77 days, Nomilin-treated mice had lower body weight, serum glucose, serum insulin, and enhanced glucose tolerance.
CONCLUSIONS:
Our results suggest a novel biological function of Nomilin as an agent having anti-obesity and anti-hyperglycemic effects that are likely to be mediated through the activation of TGR5.
Acs Symposium, 2000, 758:185-200.
Limonin and Nomilin Inhibitory Effects on Chemical-Induced Tumorigenesis.
The increased enzyme activity was correlated with the ability of these compounds to inhibit carcinogenesis.
METHODS AND RESULTS:
Nomilin was found to reduce the incidence and number of tumors per mouse of forestomach tumors induced by benzo[a]pyrene (BP). Topical application of the limonoids was found to inhibit both the initiation and the promotion phases of carcinogenesis in the skin of SENCAR mice. Nomilin appeared to be more effective at the initiation stage while limonin was more potent as an inhibitor at the promotion phase of carcinogenesis. Administration of Nomilin and limonin to the diet or by gavage inhibited BP-induced and 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone-induced lung tumor formations, respectively, in A/J mice.
CONCLUSIONS:
These findings suggest citrus limonoids are potential cancer chemopreventive agents.
HPLC of Androsin

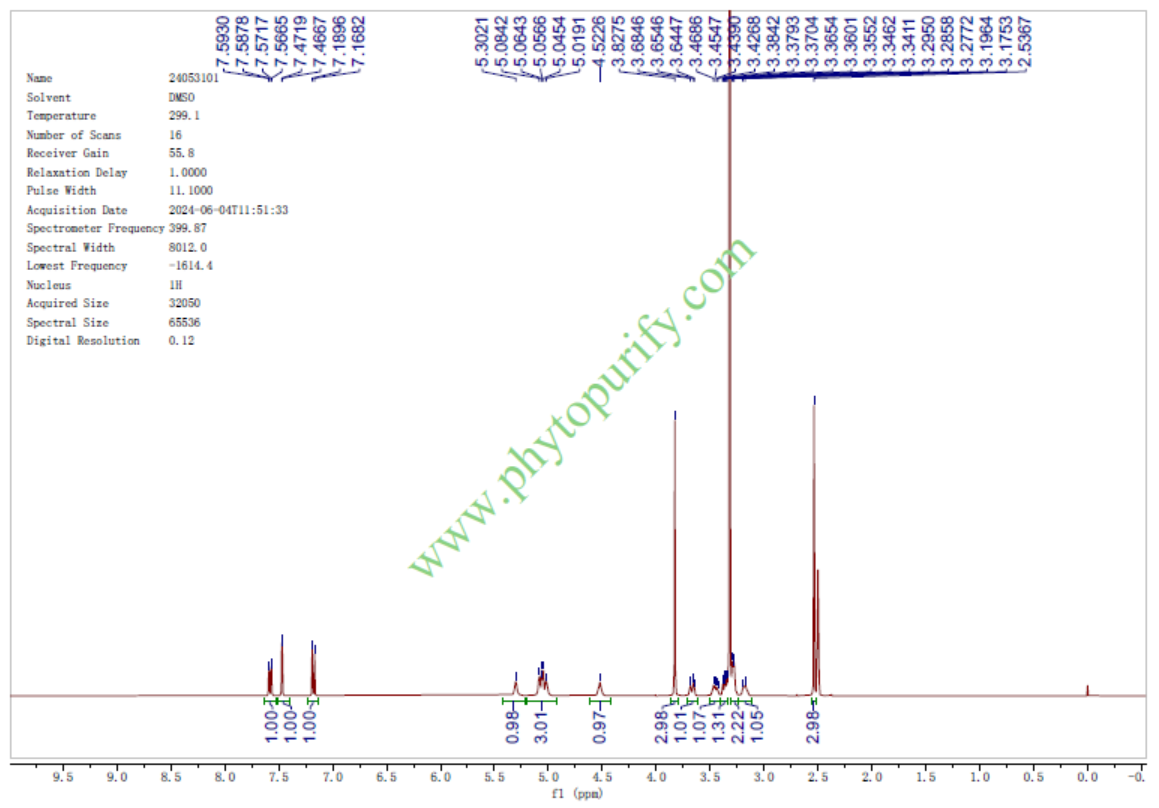
HNMR of Androsin