
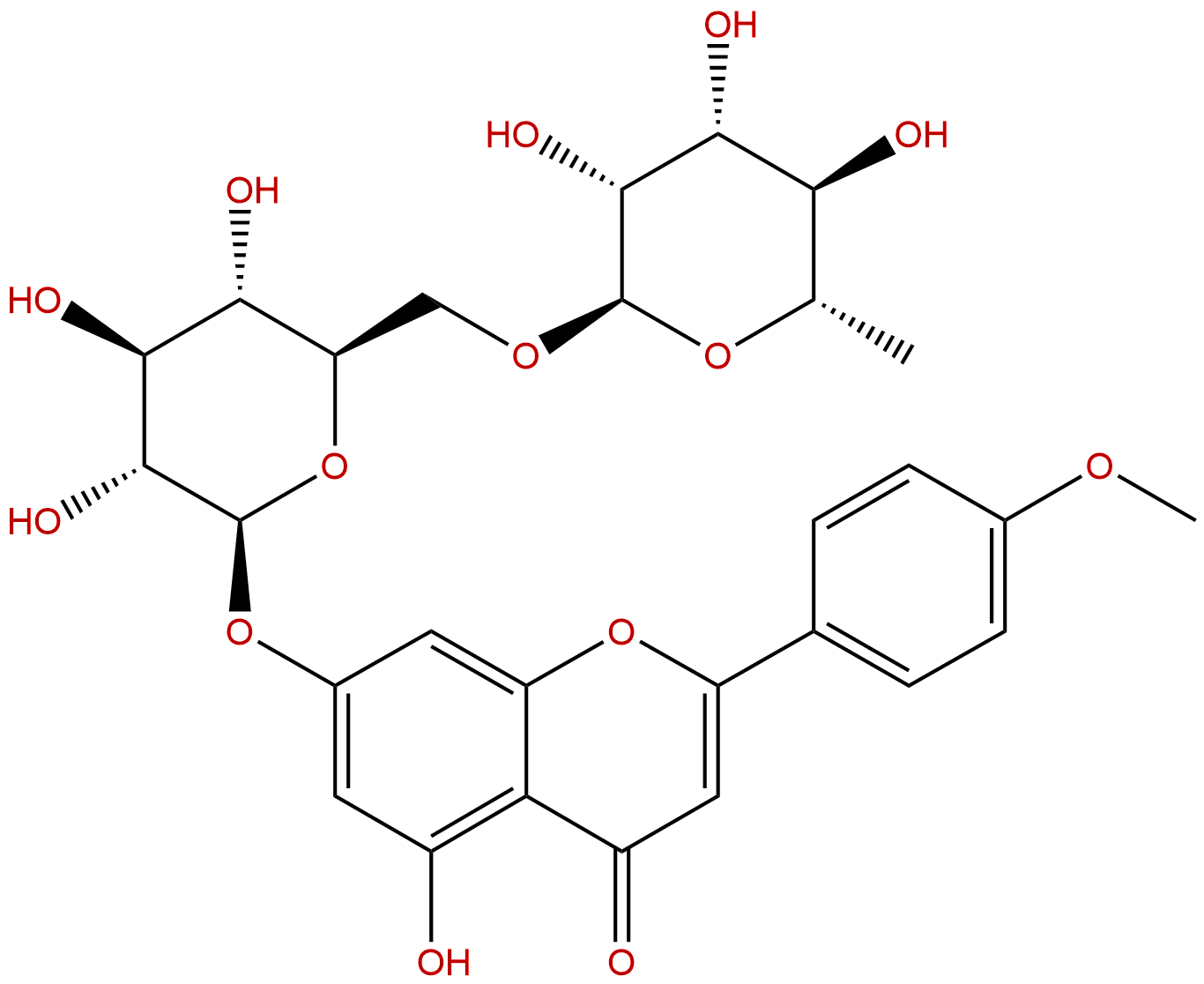
AcaciinCAS No.:480-36-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0115 |
| Formula: | C28H32O14 |
| Mol Weight: | 592.55 |
Product name: Acaciin
Synonym name: Buddleoside; linarin
Catalogue No.: BP0115
Cas No.: 480-36-4
Formula: C28H32O14
Mol Weight: 592.55
Botanical Source: Occurs in Linaria vulgaris, Robinia and Micromeria spp. and other plants
Physical Description: Yellow powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Linarin is a flavone glycoside in the plants Flos chrysanthemi indici, Buddleja officinalis, Cirsium setosum, Mentha arvensis and Buddleja davidii, possesses analgesic, antipyretic, anti-inflammatory and neuroprotective activities. [1]
Linarin is known to have anti-acetylcholinesterase effects, prevents Aβ(25-35)-induced neurotoxicity through the activation of PI3K/Akt, which subsequently inhibits GSK-3β and up-regulates Bcl-2, may be a potent therapeutic compound against Alzheimer's disease acting through both acetylcholinesterase inhibition and neuroprotection.[2]
Linarin has dose-dependent analgesic and anti-inflammatory activities.[3]
Linarin can protect osteoblasts against hydrogen peroxide-induced osteoblastic dysfunction and may exert anti-resorptive actions, at least in part, via the reduction of RANKL and oxidative damage.[4]
Linarin induces the osteogenic differentiation and mineralization of MC3T3-E1 osteoblastic cells by activating the BMP-2/RUNX2 pathway through PKA signalingin vitroand protected against OVX-induced bone lossin vivo, suggests that linarin is a useful natural alternative for the management of postmenopausal osteoporosis.[5]
References:
[1] Lou H, Fan P, Perez R G, et al. Bioorgan Med Chem, 2011, 19(13):4021-7.
[2] Martínez-Vázquez M, Ramírez Apan T O, Lastra A L, et al. Planta Medica, 1998, 64(2):134-7.
[3] Kim Y H, Lee Y S, Choi E M. Cell Immunol, 2011, 268(2):112-6.
[4] Feng X, Wang X, Liu Y, et al. Iran J Pharm Res, 2015, 14(3):949-54.
[5] Li J, Hao L, Wu J, et al. Int J Mol Med, 2016, 37(4):901-10.
[6] Guo Q, Fang H, Shen H. China Journal of Chinese Materia Medica, 2010, 35(9).
HPLC of Acaciin

HNMR of Acaciin
