
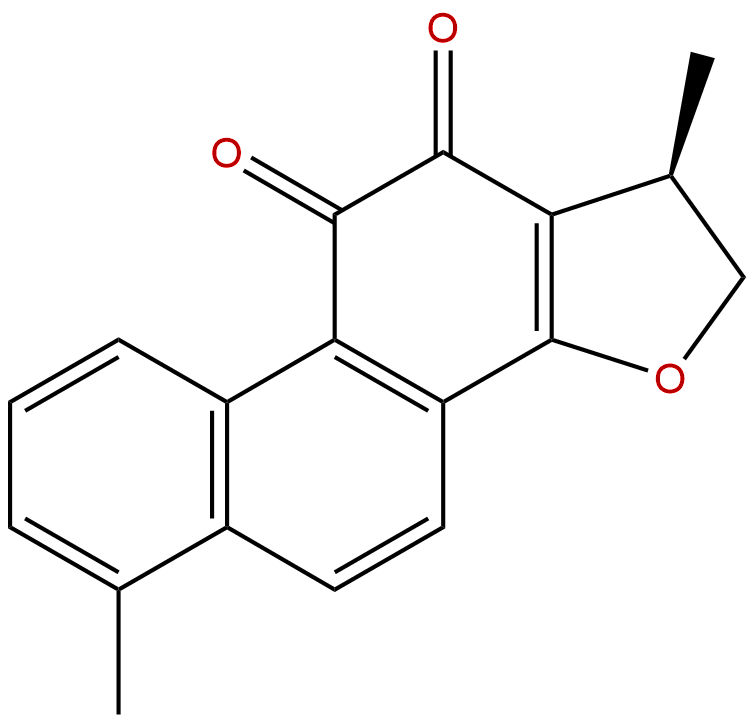
15,16-Dihydrotanshinone ICAS No.:87205-99-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0027 |
| Formula: | C18H14O3 |
| Mol Weight: | 278.307 |
Product name: 15,16-Dihydrotanshinone I
Synonym name:
Catalogue No.: BP0027
Cas No.: 87205-99-0
Formula: C18H14O3
Mol Weight: 278.307
Botanical Source: Salvia miltiorrhiza
Physical Description: Red powder
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Dihydrotanshinone I(DI) and cryptotanshinone, constituents of a medicinal plant, Salvia miltiorrhiza Bunge, have antibacterial activity against a broad range of Gram positive bacteria, they generates superoxide radicals in Bacillus subtilis lysates, the superoxide radicals are important in the antibacterial actions of the agents.[1]
Dihydrotanshinone I induces topoisomerase I-mediated DNA cleavage as strongly as camptothecin, but topoisomerase II-mediated DNA cleavage was not affected, dihydrotanshinone I inhibits the catalytic activity of topoisomerase I by the formation of a cleavable complex and at least in part through the intercalation into DNA.[2]
Dihydrotanshinone I has cytotoxicity to a variety of tumor cells, it induces a potent cytotoxicity to human umbilical vein endothelial cells with an IC 50 value of approximately 1.28 ug/ml; it could inhibit angio-genesis through suppressing endothelial cell proliferation, migration, invasion and tube formation, indicating that DI has a potential to be developed as a novel anti-angiogenic agent.[3]
Dihydrotanshinone I significantly impaires activation of extracellular signal-regulated kinase 1/2 (ERK1/2), p38 and stress-activated protein kinase/c-Jun NH2-terminal kinase (JNK/SAPK), it suppresses the growth of HeLa cells in a xenograft tumor model, which could be correlated with its modulation of TNF-α production, suggests that dihydrotanshinone I could be a valuable candidate for the intervention of NF-κB-dependent pathological conditions such as inflammation and cancer.[4]
Dihydrotanshinone I induces cell growth arrest during the S phase and subsequently, apoptosis, following its application to K562/ADR cells, exhibits cytotoxicity against various tumor cell lines. [5]
Dihydrotanshinone I combines irradiation, which can enhance apoptotic effects in human cervical cancer by HPV E6 down-regulation and caspases activation.[6]
Dihydrotanshinone I and cryptotanshinone exhibit strong inhibition towards human liver microsome (HLM)-catalyzed propofol glucuronidation, and dihydrotanshinone I exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A7.[7,8]
Dihydrotanshinone-I interferes with the RNA-binding activity of HuR affecting its post-transcriptional function.[9]
Dihydrotanshinone I induces caspase and ROS dependent apoptosis and autophagy, mediated by mitochondria in colon cancer; it could be a promising leading compound for the development of anti-tumor agent or be developed as an adjuvant drug for colon cancer therapy.[10]
Dihydrotanshinone I has inhibitory effects on CYP4A11 and CYP4F3A mediated ω-hydroxylation of arachidonic acid.[11]
References:
[1] Lee D S, Lee S H, Noh J G, et al. Biosci Biotech Biochem, 2014, 63(12):2236-9.
[2] Lee D S, Lee S H, Kwon G S, et al. Biosci Biotech Biochem, 1999, 63(8):1370-3.
[3] Weipeng, Bian, Chen, et al. Acta Bioch Bioph Sin, 2008, 40(1):1–6.
[4] Wang F, Ma J, Wang KS, et al. Int Immunopharmacol, 2015, 28(1):764-72.
[5] Lee D S, Lee S H. J Biosci Bioeng, 2000, 89(3):292-3.
[6] Ye Y, Xu W, Zhong W, et al.Mol Cell Biochem, 2012, 363(1-2):191-202.
[7] Cong M, Hu C M, Cao Y F, et al. Fitoterapia, 2013, 85(1):109-13.
[8] Gong G, Zhang S Y, Lin J J, et al. Latin Am J Pharm, 2012, 31(7):1060-3.
[9] D’Agostino V G, Lal P, Mantelli B, et al. Sci Rep, 2015, 5(3):1392-1215.
[10] Lin W, Tao H, Jing S, et al.Phytomed Int J Phytother Phytopharmacol, 2015, 22(12):1079-87.
[11] Meijuan Xu, Lifeng Jiang, Wenzheng Ju, et al. Eur J Integr Med, 2014, 6(6):735.
[12] Zhu D, Tan S. China Pharmacist, 2008, 11(03):301-3.
HPLC of 15,16-Dihydrotanshinone I

HNMR of 15,16-Dihydrotanshinone I
